294721
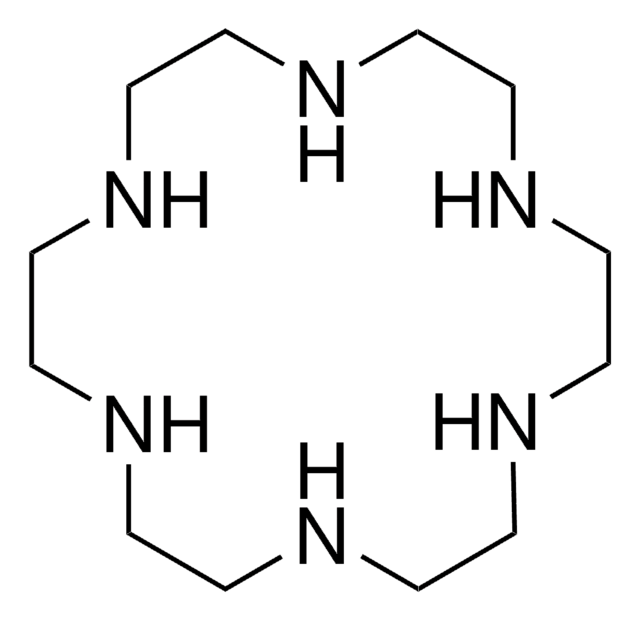
7,16-Dibenzyl-1,4,10,13-tetraoxa-7,16-diazacyclooctadecane
97%
Synonym(s):
1,10-Dibenzyl-1,10-diaza-18-crown-6, N,N′-Dibenzyl-4,13-diaza-18-crown-6
About This Item
Recommended Products
Assay
97%
form
solid
mp
80-83 °C (lit.)
SMILES string
C1COCCN(CCOCCOCCN(CCO1)Cc2ccccc2)Cc3ccccc3
InChI
1S/C26H38N2O4/c1-3-7-25(8-4-1)23-27-11-15-29-19-21-31-17-13-28(24-26-9-5-2-6-10-26)14-18-32-22-20-30-16-12-27/h1-10H,11-24H2
InChI key
WAHZGOBRKWVALN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- As a macrocycle that facilitates the modification of the electrode used in the estimation of riboflavin or vitamin B2 in food and pharmaceutical samples.
- As an ionophore in the preparation of poly(vinyl chloride) membrane-based Zn2+ sensor applicable in the estimation of Zn in water and drug samples.
- To prepare a modified graphite electrode, which is used in the detection of samarium in ores and industrial effluents.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service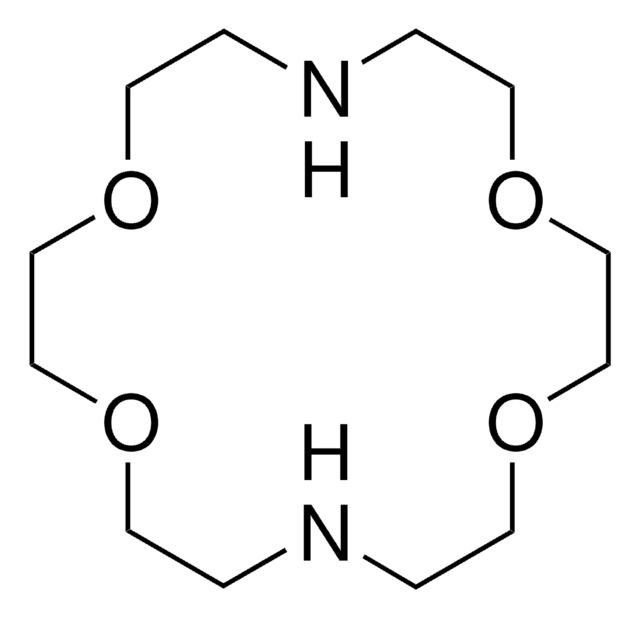
![4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane 98%](/deepweb/assets/sigmaaldrich/product/structures/189/812/8a6555e5-8de6-4236-865f-19339cee3634/640/8a6555e5-8de6-4236-865f-19339cee3634.png)
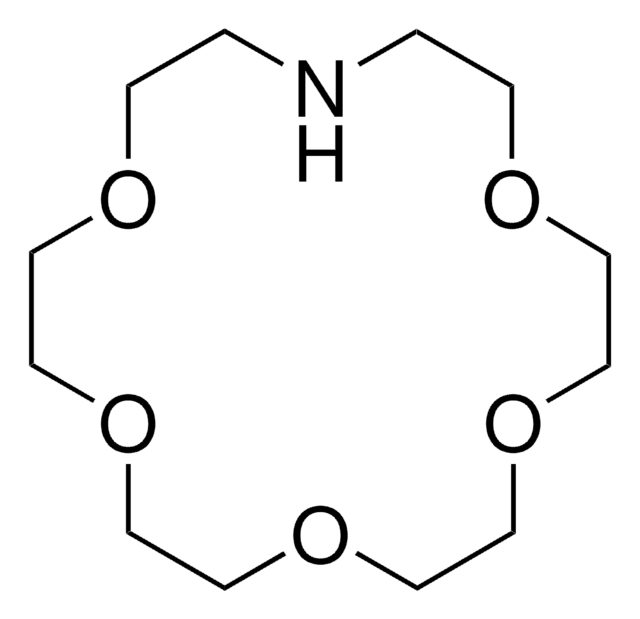
![4,7,13,16,21-Pentaoxa-1,10-diazabicyclo[8.8.5]tricosane 98%](/deepweb/assets/sigmaaldrich/product/structures/444/464/eeb08f63-862e-447e-8e41-342d713f439c/640/eeb08f63-862e-447e-8e41-342d713f439c.png)