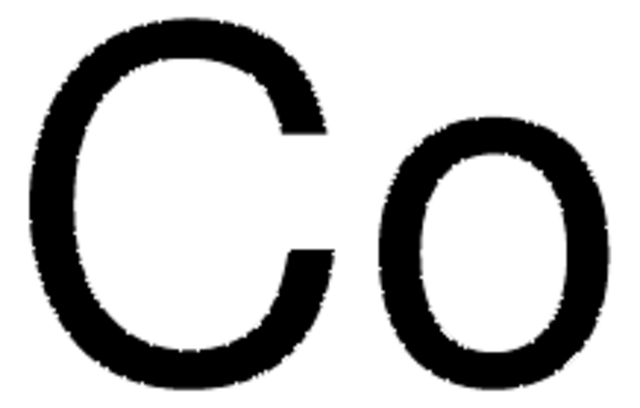266647
Cobalt
powder, <150 μm, ≥99.9% trace metals basis
Synonym(s):
Cobalt element, Cobalt-59
About This Item
Recommended Products
Assay
≥99.9% trace metals basis
form
powder
resistivity
6.24 μΩ-cm, 20°C
particle size
<150 μm
bp
2900 °C (lit.)
density
8.9 g/mL at 25 °C (lit.)
SMILES string
[Co]
InChI
1S/Co
InChI key
GUTLYIVDDKVIGB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 4 - Carc. 1B - Eye Irrit. 2 - Flam. Sol. 1 - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Biomedical implants are essentially foreign substances within the human body that must survive many years’ exposure to demanding mechanical and physiological conditions. Despite these challenges, metal implants have been widely used to substitute for or rebuild hard tissues such as bones and teeth.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service