240931
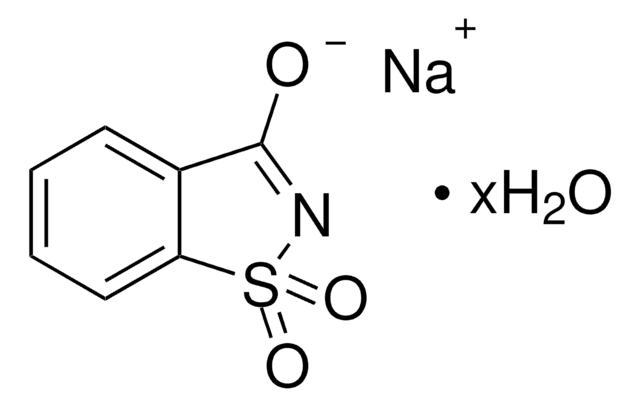
Saccharin
≥99%
Synonym(s):
2,3-Dihydroxy-1,2-benzisothiazol-3-one-1,1-dioxide, 2-Sulfobenzoic acid imide, o-Benzoic sulfimide
About This Item
Recommended Products
Assay
≥99%
form
powder
mp
226-229 °C (lit.)
solubility
acetone: 1g in 12mL(lit.)
alcohol: 1g in 31mL(lit.)
boiling water: 1g in 25mL(lit.)
water: 1g in 25mL(lit.)
SMILES string
O=C1NS(=O)(=O)c2ccccc12
InChI
1S/C7H5NO3S/c9-7-5-3-1-2-4-6(5)12(10,11)8-7/h1-4H,(H,8,9)
InChI key
CVHZOJJKTDOEJC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Gabapentin-saccharin co-crystals with enhanced physicochemical properties and in vivo absorption formulated as oro-dispersible tablets.: This study presents the formulation of gabapentin-saccharin co-crystals, demonstrating improved solubility and absorption, beneficial for pharmaceutical applications (Soliman et al., 2020, doi: 10.1080/10837450.2019.1687521).
- Improving the solubility, dissolution, and bioavailability of Ibrutinib by preparing it in a coamorphous state with saccharin.: The research focuses on enhancing the bioavailability of Ibrutinib through coamorphous preparation with saccharin, which is significant for cancer treatment (Shi et al., 2019, doi: 10.1016/j.xphs.2019.04.031).
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service