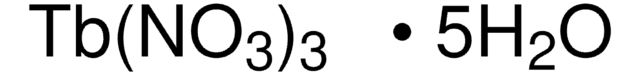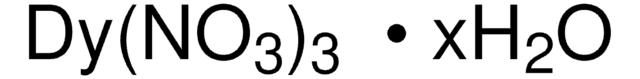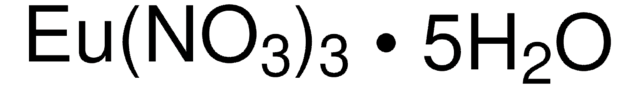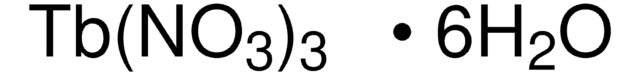229733
Lithium bromide
powder and chunks, ≥99.995% trace metals basis
Synonym(s):
Lithium monobromide
About This Item
Recommended Products
Quality Level
Assay
≥99.995% trace metals basis
form
powder and chunks
impurities
≤50.0 ppm Trace Metal Analysis
mp
550 °C (lit.)
application(s)
battery manufacturing
SMILES string
[Li+].[Br-]
InChI
1S/BrH.Li/h1H;/q;+1/p-1
InChI key
AMXOYNBUYSYVKV-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
- Space cooling using geothermal single‐effect water/lithium bromide absorption chiller: This research explores the use of lithium bromide in geothermal absorption chillers for space cooling applications (M El Haj Assad, M Sadeghzadeh, 2021).
- A facile and fast method for quantitating lignin in lignocellulosic biomass using acidic lithium bromide trihydrate (ALBTH): The paper introduces a novel method for lignin quantification using lithium bromide trihydrate (N Li, X Pan, J Alexander, 2016).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Lithium-ion batteries represent a group of electrochemical devices used for electricity storage and have attracted a lot of attention in the past two decades due to their portability, rechargeability and low cost.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service