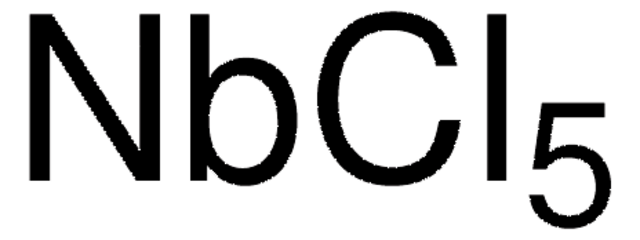215791
Niobium(V) chloride
99%
Synonym(s):
Niobium pentachloride
About This Item
Recommended Products
Assay
99%
form
powder
technique(s)
FTIR: suitable
bp
254 °C (lit.)
mp
204.7 °C (lit.)
density
2.75 g/mL at 25 °C (lit.)
SMILES string
Cl[Nb](Cl)(Cl)(Cl)Cl
InChI
1S/5ClH.Nb/h5*1H;/q;;;;;+5/p-5
InChI key
YHBDIEWMOMLKOO-UHFFFAOYSA-I
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- As a precursor to prepare K2NbF7:Mn4+ red phosphors for white light-emitting diode(LED) devices.
- As a Lewis acid to synthesize fluorescein dye derivatives for dye-sensitized solar cells.
- As a dopant to enhance the thermoelectric properties of n-type tin selenide.
- As a precursor to synthesize Nb2O5 microspheres and TiNb2O7 nanoparticles as high-rate capability and long-cycle-life anode materials for lithium-ion batteries .
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service