About This Item
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.551 (lit.)
bp
108-110 °C/11 mmHg (lit.)
233 °C (lit.)
density
0.963 g/mL at 25 °C (lit.)
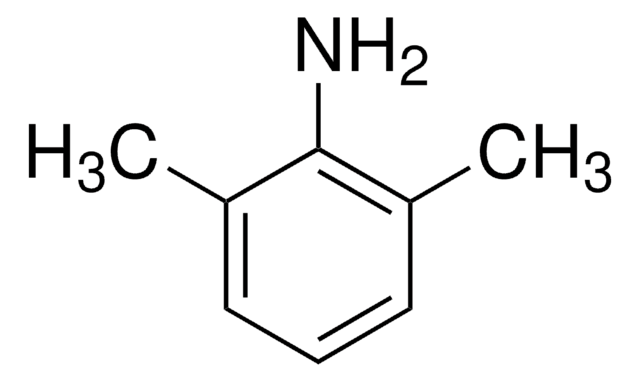
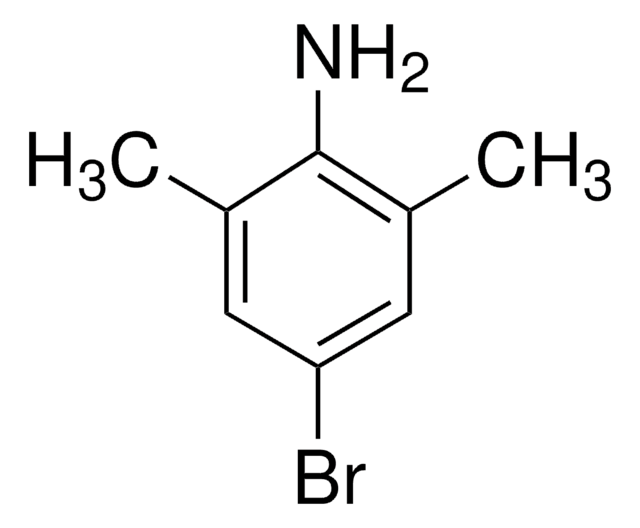
SMILES string
Cc1cc(C)c(N)c(C)c1
InChI
1S/C9H13N/c1-6-4-7(2)9(10)8(3)5-6/h4-5H,10H2,1-3H3
InChI key
KWVPRPSXBZNOHS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Synthesis and crystal structures of N,2,4,6-tetra-methyl-anilinium tri-fluoro-methane-sulfonate and N-iso-propyl-idene-N,2,4,6-tetra-methyl-anilinium tri-fluoro-methane-sulfonate.: This study presents the synthesis and structural analysis of two compounds derived from 2,4,6-Trimethylaniline, contributing to understanding its role in organic synthesis and crystal engineering (Stewart et al., 2024).
- Analysis of aromatic amines in human urine using comprehensive multi-dimensional gas chromatography-mass spectrometry (GCxGC-MS).: This paper explores advanced analytical techniques for detecting aromatic amines, including 2,4,6-Trimethylaniline, demonstrating its relevance in environmental and biological studies (Lorenzo-Parodi et al., 2024).
- Efficient Rhodium-Catalyzed Multicomponent Reaction for the Synthesis of Novel Propargylamines.: This research highlights the use of 2,4,6-Trimethylaniline in developing new catalytic methods, showcasing its importance in pharmaceutical intermediate synthesis (Rubio-Pérez et al., 2015).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service