129445
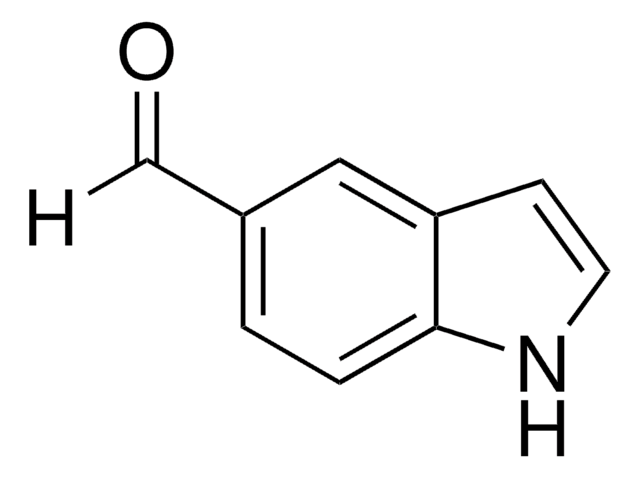
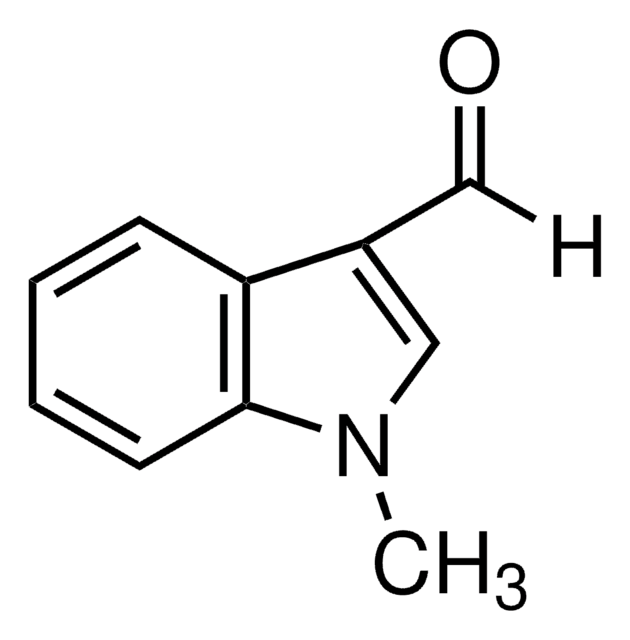
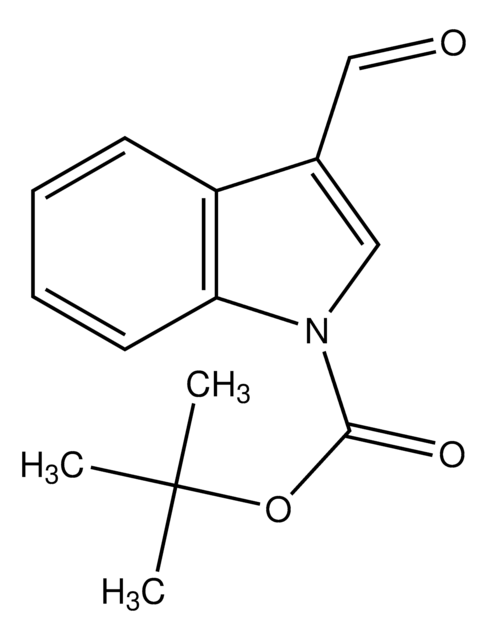
Indole-3-carboxaldehyde
97%
Synonym(s):
β-Indolylaldehyde, 3-Formylindole, 3-Indolylformaldehyde, Indole-3-carbaldehyde, NSC 10118
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H7NO
CAS Number:
Molecular Weight:
145.16
Beilstein:
114117
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
193-198 °C (lit.)
SMILES string
O=Cc1c[nH]c2ccccc12
InChI
1S/C9H7NO/c11-6-7-5-10-9-4-2-1-3-8(7)9/h1-6,10H
InChI key
OLNJUISKUQQNIM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Indole-3-carboxaldehyde can undergo Schiff bases condensation to form multifunctional silica nano-vehicles and magnetic nanoparticles.
Application
Indole-3-carboxaldehyde was used to prepare analogs of the indole phytoalexin cyclobrassinin with NR1R2 group. It was also used as the starting material for the synthesis of higher order indoles including isoindolo[2,1-a]indoles, aplysinopsins, and 4-substituted-tetrahydrobenz[cd]indoles.
Reactant for preparation of:
- Analgesic agents
- Hypoglycemic agents
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Antibacterial and antifungal agents
- Antiamoebic and cytotoxic agents
- Inhibitors of the Dengue virus protease with antiviral activity in cell-culture
- Curcumin analogues as possible anti-proliferative & anti-inflammatory agents
- Inhibitors of Bcl-2 family proteins
- Inhibitors of the C-terminal domain of RNA Polymerase II as antitumor agents
- Inhibitors of TNF-α and IL-6 with anti-tubercular activity
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Qiu-Yun Chen et al.
Colloids and surfaces. B, Biointerfaces, 114, 158-163 (2013-11-05)
Multifunctional silica nano-vehicles (SiO2@indol-IL) and magnetic nanoparticles (Fe3O4@indol-IL) were constructed through the Schiff bases condensation of indole-3-carboxaldehyde and 4-acetyl-N-allyl pyridinium chloride (ILs) with the amine groups of silica and magnetic nanoparticles. SiO2@indol-IL can inhibit the proliferation of HepG-2 cells in
Synthetic Communications, 23, 55-55 (1993)
Heterocycles, 38, 1479-1479 (1994)
Mariana Budovská et al.
Bioorganic & medicinal chemistry, 21(21), 6623-6633 (2013-09-10)
An effective synthesis of analogs of the indole phytoalexin cyclobrassinin with NR1R2 group instead of SCH3 was developed starting from indole-3-carboxaldehyde. The target compounds were prepared by spirocyclization of 1-Boc-thioureas with the formation of isolable spiroindoline intermediates, followed by the
Indian J. Chem. B, 33, 4-4 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service