101834
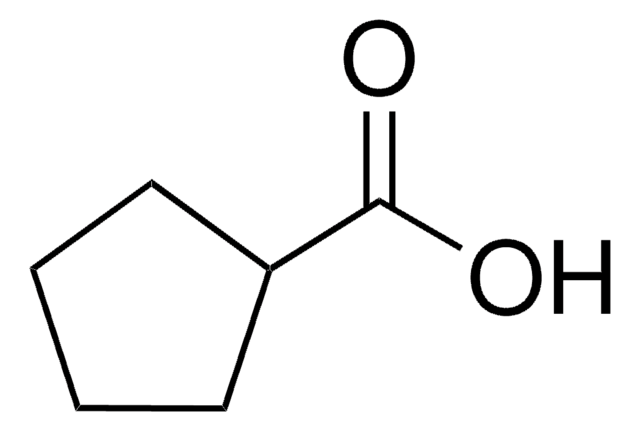
Cyclohexanecarboxylic acid
98%
Synonym(s):
Hexahydrobenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H11CO2H
CAS Number:
Molecular Weight:
128.17
Beilstein:
970529
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
refractive index
n20/D 1.461 (lit.)
bp
232-233 °C (lit.)
mp
29-31 °C (lit.)
solubility
H2O: soluble 0.201g in 100g at 15 °C
organic solvents: soluble
density
1.033 g/mL at 25 °C (lit.)
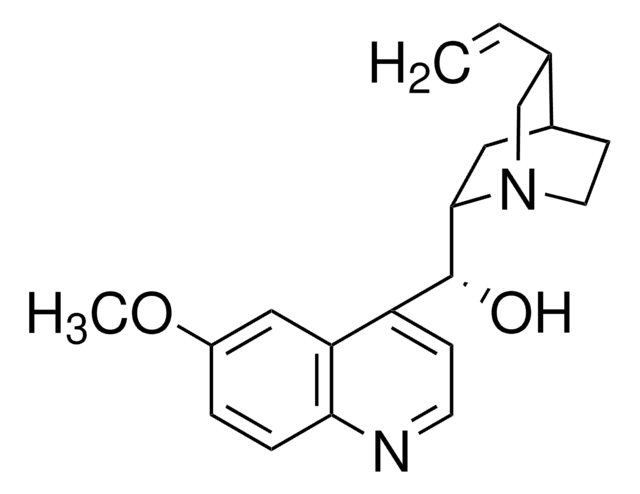
SMILES string
OC(=O)C1CCCCC1
InChI
1S/C7H12O2/c8-7(9)6-4-2-1-3-5-6/h6H,1-5H2,(H,8,9)
InChI key
NZNMSOFKMUBTKW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Cyclohexanecarboxylic acid was used in a study to determine complex binding constants of the three native cyclodextrins with seven cyclohexane derivatives.
Biochem/physiol Actions
Cyclohexanecarboxylic acid undergoes microbial degradation by a strain of Antherobacter to form para-hydroxybenzoic acid. Cyclohexanecarboxylic acid undergoes aromatization and converts to Hippuric acid in rat liver extracts in vitro. Cyclohexanecarboxylic acid is the starting reagent for the synthesis of polyketide-type antibiotics, Phoslactomycins.
Preparation Note
0.201g of cyclohexanecarboxylic acid dissolves in 100g water at 15°C.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The microbial degradation of cyclohexanecarboxylic acid: a pathway involving aromatization to form p-hydroxybenzoic acid.
Blakley ER.
Canadian Journal of Microbiology, 20(10), 1297-1306 (1974)
A Gadre et al.
Journal of pharmaceutical sciences, 86(2), 236-243 (1997-02-01)
Complex binding constants of the three native cyclodextrins with seven cyclohexane derivatives (all possessing the carboxylic acid group) and with the series C6H5(CH2)nCOOH (n = 0 to 4) were measured in aqueous solution at 25 degrees C by potentiometry and
Biosynthesis of phoslactomycins: cyclohexanecarboxylic acid as the starter unit.
Sekiyama Y, et al.
Tetrahedron, 59(38), 7465-7471 (2003)
Aromatization of cyclohexanecarboxylic acid.
B M Babior et al.
The Journal of biological chemistry, 241(16), 3643-3651 (1966-08-25)
Janice C Paslawski et al.
Biodegradation, 20(1), 125-133 (2008-07-18)
Naphthenic acids are a complex mixture of organic compounds which naturally occur in crude oil. Low molecular weight components of the naphthenic acids are known to be toxic in aquatic environments and there is a need to better understand the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service