All Photos(3)
About This Item
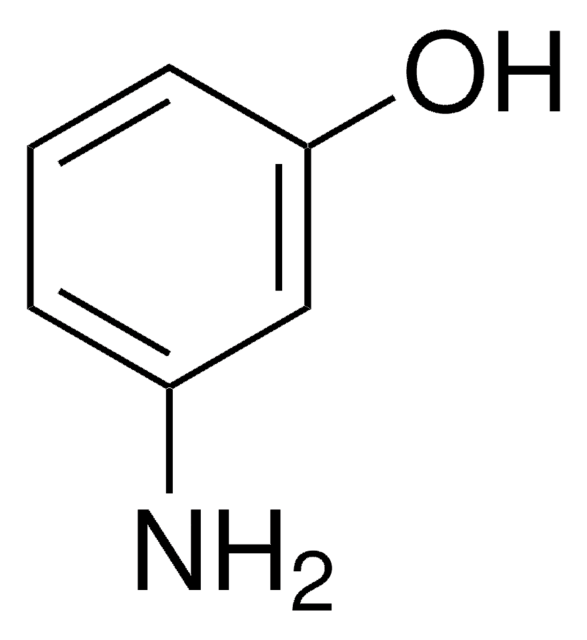
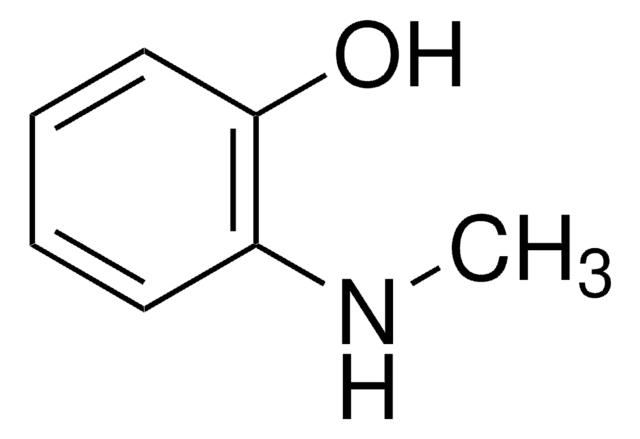
Linear Formula:
H2NC6H4OH
CAS Number:
Molecular Weight:
109.13
Beilstein:
636059
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
164 °C/11 mmHg (lit.)
mp
120-124 °C (lit.)
SMILES string
Nc1cccc(O)c1
InChI
1S/C6H7NO/c7-5-2-1-3-6(8)4-5/h1-4,8H,7H2
InChI key
CWLKGDAVCFYWJK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3-Aminophenol has been used in the synthesis of disulfonated bis[4-(3-aminophenoxy)phenyl]sulfone (S-BAPS).
It can be used to synthesize:
It can be used to synthesize:
- Methyl 2-oxo-7-[(triphenylphosphoranylidene)amino]-2H-chromene-4-carboxylate by reacting with dimethyl acetylenedicarboxylate (DMAD) in the presence of triphenylphosphine.
- 3-Amino-2-cyclohexen-1-one via palladium-catalyzed hydrogenation.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yingchun Gu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 213, 263-271 (2019-02-01)
Filamentous bacteria, particularly Microthrix parvicella, are mainly responsible for bulking or foaming of activated sludge. Based on the affinity of M. parvicella to the hydrophobic characteristics of long-chain fatty acids, a novel bisoctyl rhodamine B (BORB) and a novel fluorescence
A Practical and One-Pot Procedure for the Synthesis of 3-Amino-2-cyclohexen-1-one from 3-Aminophenol.
Sajiki H
Organic Process Research & Development, 9(2), 219-220 (2005)
Sijing Chen et al.
Polymers, 11(6) (2019-06-20)
Benzoxazine containing fluorinated aromatic ether nitrile linkage (FAEN-Bz) had been synthesized from 2,6-dichlorobenzonitrile, 4,4'-(hexafluoroisopropylidene)diphenol (bisphenol AF), 3-Aminophenol, formaldehyde, phenol by condensation polymerization and Mannich ring-forming reaction. Structures of the monomer were verified by Proton NMR spectrum (1H-NMR) and Fourier transform
Vinyltriphenylphosphonium salt mediated synthesis of 1, 4-benzoxazine and coumarin derivatives.
Yavari I, et al.
Tetrahedron, 58(34), 6895-6899 (2002)
Ping-Chih Hsu et al.
Oncology reports, 42(2), 697-707 (2019-06-25)
Human non‑small cell lung cancer (NSCLC) is associated with an extremely poor prognosis especially for the 40% of patients who develop brain metastasis, and few treatment strategies exist. Cucurbitacin E (CuE), an oxygenated tetracyclic triterpenoid isolated from plants particularly of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service