Seamless Integration of Glucose Control in CHO Culture Using Raman Spectroscopy
Process analytical technology (PAT) and quality by design (QbD) guidelines, published by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), emphasize that quality cannot be tested into a product but must be deployed throughout process development. These approaches are being leveraged in the biopharmaceutical industry to ensure quality is designed into processes and to achieve quality improvements.
Monitoring critical quality attributes (CQA), such as glycosylation is important in ensuring the stability, immunogenicity, safety, and potency of biomolecules. Maintaining the glucose concentration at a steady level in the bioreactor is essential to control and optimize the process yield and quality, including glycosylation.1,2 Manual sampling and feeding of the bioreactor, however, are costly and time consuming and increase the risk of contamination each time the sterile boundary is penetrated.
Monitoring and Controlling Glucose Concentration in Cell Cultures
In this study, a ProCellics™ Raman Analyzer with Bio4C® PAT Raman Software (Raman PAT Platform) was used to implement a feedback control loop in a CHO cell culture process to monitor glucose concentration and enable maintenance of stable glucose levels without the need for manual intervention. The ability to monitor and maintain the desired glucose concentration led to improved process quality and supports proper glycosylation of the drug product.
The feedback control loop was based on a direct Open Platform Communications United Architecture (OPC-UA) connectivity between the Raman PAT platform and the bioreactor control system.
FreeStyle™ CHO-S (Gibco®) cells were cultivated in CD-CHO medium (Gibco®) with 8 mM glutamine, 1‰ anti-clumping agent (Gibco®) and 0.5% penicillin/ streptomycin in a 3 L glass bioreactor. The bioreactor was inoculated with cells at a density of 0.4 × 106 cells/mL in a starting volume of 2 L. Bioreactor settings are provided in Table 1.
For the monitored batch, the culture was fed with a 15% v/v CHO CD EfficientFeed™ B (Gibco®) feed solution on day zero. Glutamine was added when the concentration dropped below 4 mM; constant glutamine feeding began on day three. For glucose feeding, a control loop was programmed based on the desired glucose concentration. The pump rate of the complex feed solution was controlled by a normal law (on DASware® control 5) based on the glucose concentration read by the ProCellics™ Raman Analyzer probe to maintain a glucose concentration of 5 g/L. The communication was integrated via OPC-UA and the function was defined as:
Pump rate = 2,000e (- [Glucose]2/5)
Control cultures were fed using CHO CD EfficientFeed™ B (Gibco®) with 15% v/v on day 0 and 10% v/v on day 3, 6 and 9. When the glucose concentration dropped below 4 g/L, a highly concentrated glucose solution was added. Glutamine was also added when the concentration dropped below 4 mM in addition to a constant feed of glutamine starting on day 3.
Model building was used to correlate the reference values obtained by an off-line analyzer (Nova Biomedical FLEX2) and the ProCellics™ Raman Analyzer. The spectra were preprocessed on the Bio4C® PAT Raman Software (SNV on the water region, Savitzky Golay derivative with 3 points (15 cm-1, polynomial order 2nd and 1st derivative) and spectral selection (350–1,775 cm-1 + 2,800–3,000 cm-1)) to create a data set. The reference values were automatically linked to their corresponding spectra. The chemometric models for the monitoring were based on four standard fed-batch cultures (with a total of 103 points). A Partial Least Squares (PLS) model was developed for each monitored parameter using multivariate analytics modeling software. Models for viable cell density (VCD), glucose and lactate were created. The ProCellics™ Raman Analyzer with Bio4C® PAT Raman Software acquired and preprocessed the Raman spectra and calculated the process parameters including glucose concentration.
Measurements were taken every 30 minutes and the pump rate adjusted as needed. Communication between Bio4C® PAT Raman Software and DASware® control 5 software was enabled by an OPC-UA connectivity.
Precise Maintenance of Glucose Concentration using the Raman PAT Platform
Glucose was consumed by the cells and stabilized at 5 g/L. As shown in Figure 1A, the glucose concentration was precisely maintained at 5 g/L for 3 days by the programmed feedback loop. As a control to compare the accuracy of the Raman analyzer measurements to traditional off-line sampling methods, daily, off-line samples were collected to measure glucose concentration.
Feeding was stopped when the maximum vessel volume was reached, and the process was stopped when the glucose concentration dropped below 1 g/L. A classical fed-batch process with manual glucose addition was performed as a control (Figure 1B). Cell growth kinetics and the maximum cell density in the feedback-controlled run were comparable with the classical fed-batch run. However, the lactate concentration in the feedback-controlled run was lower (1.8 g/L) in comparison to the control run (2.8 g/L). This is a noteworthy result as a high lactate concentration can be toxic for cells. Figure 2 shows the culture parameters as displayed by the Bio4C® PAT Raman software.

Figure 1Real-time monitoring of glucose, lactate and VCD for a fed-batch culture with a glucose feedback control loop strategy (A) and for a fed-batch culture with a manual glucose addition (B).
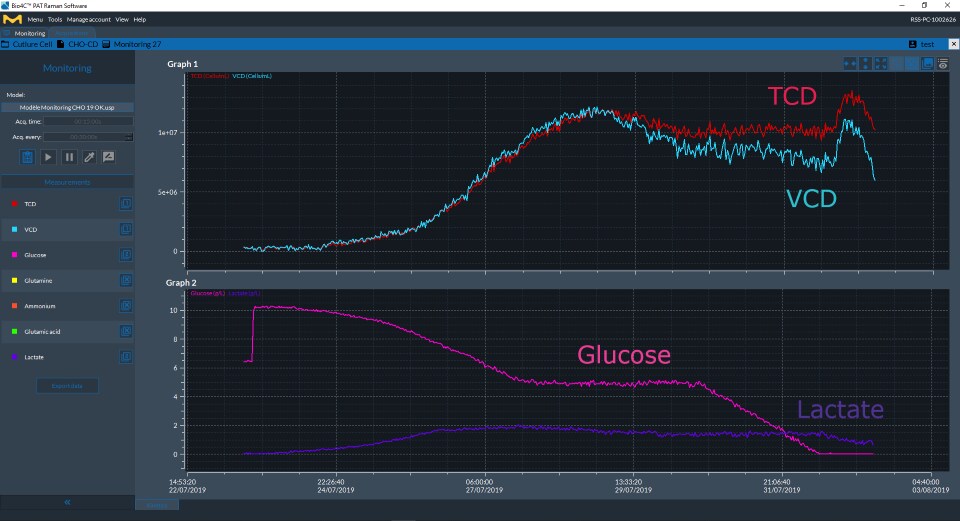
Figure 2Cell culture parameters (glucose, lactate, TCD and VCD) measured over the duration of the bioreactor culture as displayed in real time on Bio4C® PAT Raman Software.
Data monitored by the Bio4C® PAT Raman Software were efficiently and easily communicated to the DASware® control 5 software via an OPC-UA protocol. Following setup, automation of the feedback control loop was complete and reliable.
With use of the feedback control loop, the glucose concentration was steadily maintained for three days, and process performances were similar to those of regular fed-batch cultures. The process was completely automated for glucose concentration management and did not require any manual intervention to sample the bioreactor, reducing the risk of contamination. In addition, this approach reduces the risk of batch failures due to a lack of glucose resulting from gaps in manual monitoring such as during the night or on weekends.
Learn more about PAT
References
To continue reading please sign in or create an account.
Don't Have An Account?