Drospirenone Assay - USP Monograph
Eddy Tan, Associate Senior Scientist
Introduction
Drospirenone is a progestin medication that is used in birth control pills to prevent pregnancy and in menopausal hormone therapy. The Purospher® STAR RP-18e Hibar® HPLC column, 250 x 4.6 mm, 3 µm is used to demonstrate the assay of drospirenone following the USP monograph.1
Figure 1.Drospirenone
Experimental Conditions
Standard Preparation
- Add 25 mL of acetonitrile gradient grade and 25 mL of water into a graduated cylinder. Mix well - this is the acetonitrile:water (1:1) diluent.
- Weigh ~25 mg of drospirenone CRM into a 5 mL volumetric flask.
- Add ~4 mL of diluent to it and sonicate for 5 min.
- Top-up to mark with diluent and mix well. This is the drospirenone stock standard.
- Dilute to 0.3, 0.4, 0.5. 0.6, 0.7, 0.8 and 0.9 mg/mL with diluent.
Standard solution for relative standard deviation and tailing factor test: 0.6 mg/mL drospirenone in diluent
Standard concentrations for linearity, LOD and LOQ tests: 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 mg/mL in diluent
HPLC Method
The used HPLC conditions are shown in Table 1.
Results and Discussion
System Suitability
Acceptance Criteria for Standard Solution:
- Tailing factor: 0.8 to 1.5
- Relative Standard Deviation: NMT 2.0%
The peak tailing factor is 1.10 (Table 2) and the % RSD of drospirenone is 0.2% (Table 3); both criteria are within the specifications defined in the monograph.
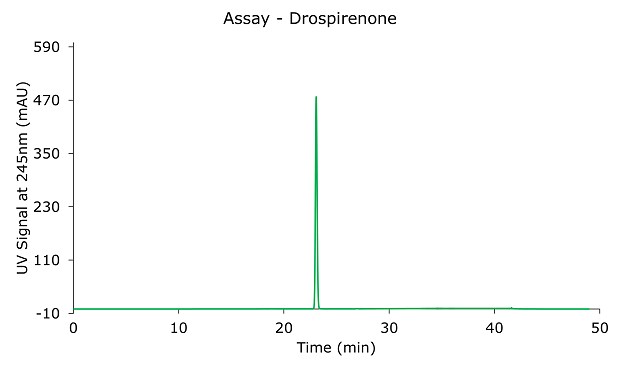
Figure 1.Drospirenone 0.6 mg/mL standard solution.
Linearity, Limit of Detection (LOD) and Limit of Quantitation (LOQ)
The Limit of Detection (LOD) for drospirenone is 0.017 mg/mL and the Limit of Quantification (LOQ) is 0.052 mg/mL. The range from 0.3 to 0.9 mg/mL is linear with the R2 as 0.9995 (Table 4).
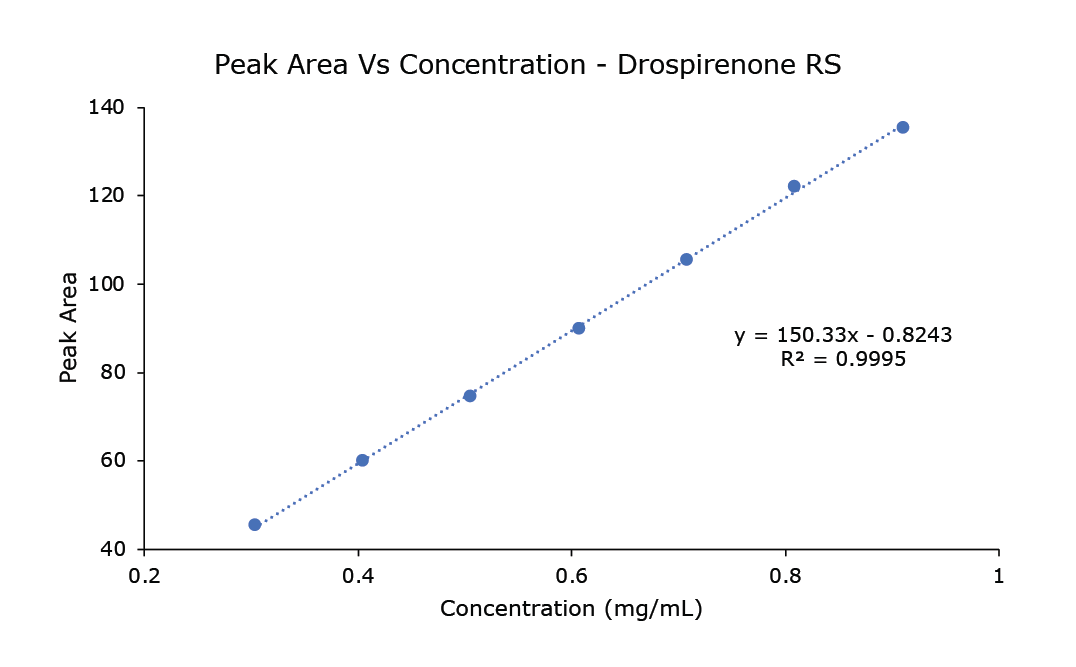
Figure 2.Linearity curve from 0.3 to 0.9 mg/mL of drospirenone.
Conclusion
The Purospher® STAR RP-18e Hibar® RT column is able to comply with the system suitability criteria indicated in the USP monograph for drospirenone using the described method. This column is therefore suitable for the determination following the USP monograph.
References
To continue reading please sign in or create an account.
Don't Have An Account?