Sterility Testing of Products to be Diluted in Isopropyl Myristate
Sterility Testing of Viscous Oils, Water-in-Oil Emulsions and Fatty Base Ointments
Membrane filtration is the pharmacopeia method of choice for sterility testing. Viscous products, such as creams and ointments, can be difficult to filter and are normally diluted in a sterile solvent, such as Isopropyl Myristate (IPM). Testing such products may be problematic if specific testing procedures are not implemented and appropriate devices are not used. The ‟Green Base” Steritest® NEO device is the perfect choice for testing solvents, creams, ointments, and veterinary injectables due to its solvent-resistant and IPM-resistant nylon canister, Durapore® (PVDF) membrane, reinforced base structure and canister connection.

Figure 1.Isoproply myristate and Steritest® NEO canister.
The new Steritest® NEO device is upgraded with new improvements such as colored clamps, graduations for accurate volume measurement, optimized identification, and traceability with the new peel-off label. The test system offers an optimized and fully compliant testing process, when used with the Steritest® Symbio pump, specific accessories and high-quality culture media and rinsing fluids.
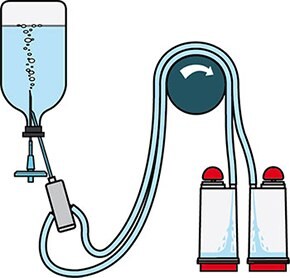
Figure 2.Schematic diagram of sterility testing using Steritest® NEO devices.
Sterility Testing Experimental Materials
- Membrane filtration device: Green base Steritest® NEO device, TZHVSL210
- Solvent: Isopropyl Myristate (IPM) sterile, ready-to-use solvent, 1466280006
- Culture media: Soybean–Casein Digest medium (SCDB), Fluid Thioglycollate Medium (FTM), and Clear Fluid Thioglycollate Medium (CTM)
- Rinse fluids: Fluid A, Fluid D, and Fluid K (double packaged media and fluids with different formats are available).
- Isopropyl alcohol (IPA) as a disinfecting agent
- Steritest® Symbio Pump for laminar flow hood or isolator.
Sterility Testing Method and Sample Preparation
The following procedure is an example of a test method that will serve as a base during method development. It should be validated afterward following the instructions mentioned in pharmacopeia before use in routine.
- Set up the Steritest® pump and device system
- Warm IPM solvent and Fluid K rinsing fluid bottles to a maximum of 44 °C
- Take one bottle of warm IPM. Wipe the bottle with 70% IPA before placing it in the testing environment
- Aseptically dispense the appropriate amount of product as required by the USP/EP/JP into the bottle of warm IPM
- Take the remaining IPM bottles, one at a time, wiping each bottle before placing it in the testing environment
- Pre-wet each membrane with 50 mL of warm IPM
- Filter the diluted product
- Rinse each membrane three times with 100 mL of warmed Fluid K (600 mL total per Steritest® NEO unit)
- Fill each canister with 100 mL Fluid K and then filter the fluid.
- When approximately 30 to 50 mL of Fluid K have passed through the membrane, gently swirl the canister to rinse residuals of IPM from the canister sides
- Then allow all the Fluid K to filter through the membrane. Repeat this procedure two additional times with Fluid K
- Do the last rinse with 100 mL of Fluid A per membrane to remove residuals of Fluid K
- Add growth media Soybean–Casein Digest medium (SCDB) and Clear Fluid Thioglycollate Medium (CTM) to each respective canister and start incubation (SCDB at 20 to 25 °C for 14 days; FTM at 30 to 35 °C for 14 days)
- Observe for growth/no growth at regular intervals during incubation
Pharmacopoeia Acceptance Criteria and Method Suitability Test
According to pharmacopeias, method suitability must be demonstrated. Inoculate the last fluid rinse with not more than 100 CFU using microorganisms recommended in the pharmacopeias. Environmental isolates can also be tested. When the filtration is complete, add media (SCDB and FTM) to the canisters and incubate for the proper time and temperature, following pharmacopeia recommendations. Then growth is observed in the canisters, then the pharmacopeia acceptance criteria are met.
Note: In case of direct inoculation of microorganisms into IPM, the addition of 9% of NaCl peptone buffer ensures recovery rate above 50%.
For further reading and more information click on the links below
Method Development and Validation Services
Our team of expert engineers will assist you in implementing method development and validation within your QC microbiology lab. Additionally, you will be provided with basic and advanced technical training on sterility testing, on-site or remotely.
Rely on our expertise to support you in various situations including:
- New laboratory sterility testing equipment
- New product or reformulated product sterility testing
- Compliance with updated regulations: EP, USP, JP, etc.
- PQ consultancy after the completion of IQ/OQ
- Method development execution or consultancy
- Investigation of out-of-specification results
To continue reading please sign in or create an account.
Don't Have An Account?