Viable but Nonculturable (VBNC) Bacteria
Jvo Siegrist
Microbiology Focus Edition 1.4
Culturing is one of the fundamental steps in microbiology. The plate count technique is a standard culturing method to enumerate viable bacteria. However, many bacterial cells could enter a distinct state called the viable but non-culturable (VBNC) state. The term “viable but nonculturable” or “not immediately culturable” describes cells that cannot normally be cultured. In most cases, the non-spore-forming bacteria are in a survival state (e.g., resting, dormancy, quiescence, or debilitation) with the metabolic pathways still being active, but the organisms are not growing.
- Challenges in Detecting Viable but Nonculturable Bacteria by Common Culture Methods
- Application of Ferrioxamine E in the Growth and Recovery of VBNC Bacteria
- Related Products Assisting in VNBC Detection
We offer ready-to-use agar plates, liquid media bottles, tubes, or ampoules, broths, and rinse fluids for your microbial testing application.
Challenges in Detecting Viable but Nonculturable Bacteria by Common Culture Methods
According to the latest definition, VBNC cells are regarded as viable and potentially replicative, but the methods required for resuscitation are beyond our current knowledge. With special media or with certain supplements it has been shown to recover them. VBNC bacteria have often undergone a treatment like heating, drying, setting under high osmotic pressure (high salt content), or contact with inhibiting chemicals. The treatment resulted in sensitive cells or sub-lethally damaged cells, which can be caused by the loss of some ribosomes, damaged enzymes, cell membranes, and other problems causing malfunctions in cells.
In recent years many species of Vibrio cholerae, E. coli, Campylobacter jejuni, Salmonella spp., Listeria monocytenenes, and Yersinia enterocolitica have been reported to be viable but non-culturable (VBNC) state. [1-10] It is important to note that bacteria that enter the VBNC state may become culturable again, and thus this state may be reversibly called “resuscitation” to describe the recovery of non-culturable cells. It was found that resuscitation can be mediated by a physical stimulus like temperature upshift and different kinds of chemical stimuli, including gas mixtures, amino acids, rich media, Supplements, etc. The addition of supplements to the re-enriched or enriched media has enabled to improvement of the recovery of many VBNC cells.
Application of Ferrioxamine E in the Growth and Recovery
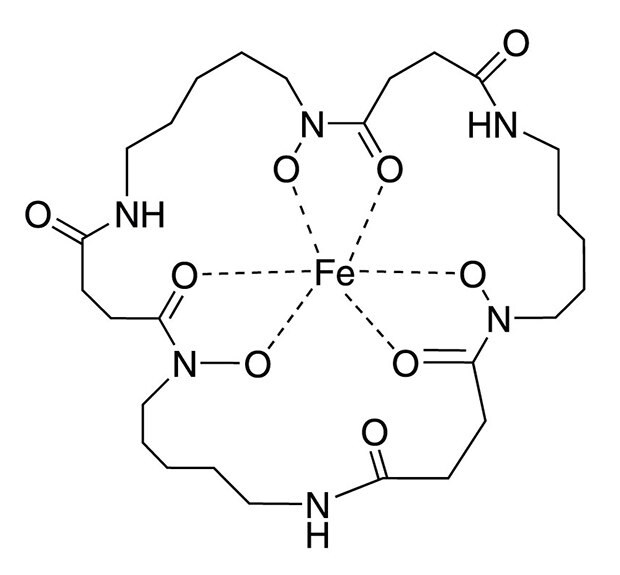
Structure of Ferrioxamine E
Ferrioxamine E, an organo-metallic natural trihydroxamate siderophore, is used as an effective growth factor in culturing many VBNC cells. Supplementing the pre-enrichment and enrichment broths with ferrioxamine E significantly improved the recovery of Salmonella, Cronobacter spp., Staphylococcus aureus, and Yersinia enterocolitica from artificially or naturally contaminated foods [1-3]. A concentration of ferrioxamine E in the range of 5-200 ng/mL supports growth (Table 1). Ferrioxamine E provides the essential micro-nutrient iron (III) to the organisms. This leads to a reduced lag phase in the medium and reactivates damaged bacteria.
Ferrioxamine E in Buffered Peptone Water is the recommended media of choice by the ISO-Norms for Enterobacteriacea. The motility of Salmonella is also improved, enabling their identification by semisolid selective motility media like MRSV, DIASSALM, or SMS.
Ferrioxamine E acts as a semi-selective agent in isolating small quantities of cells from dried powders like tea, spices, dried fruits, etc., as it does not improve the growth of E. coli, Shigella, Proteus, Providencia, and Morganella species. Ferrioxamine E with Desferrioxamine B is used as an enrichment media that enables fast and selective detection of methicillin-resistant Staphylococcus aureus (MRSA). Desferrioxamine B adsorbs iron traces and thus inhibits the growth of concomitant microorganisms, and Ferrioxamine E supports the growth of Staphylococcus aureus.
References
To continue reading please sign in or create an account.
Don't Have An Account?