Polymeric Antioxidants and Nanobiomaterials for Innovative Therapeutic Modalities
Ilker S. Bayer
Smart Materials, Istituto Italianao di Tecnologia, Via Morego 30 Genova, 16163, Italy
Material Matters™, 2022, 17.2 | Material Matters™ Publications
Introduction
Oxidative stress is a critical element of associating environmental toxicity with the multistage carcinogenic process. Reactive oxygen species (ROS) or reactive oxygen and nitrogen species (RONS) are generated in response to both endogenous (from within) and exogenous (outside) stimuli. To counterbalance RONS-mediated damage, an endogenous antioxidant defense system is activated. However, when the degree of oxidation exceeds such preventive mechanisms, oxidative stress arises. Chronic and cumulative oxidative stress induces various modifications to essential biological macromolecules such as DNA, lipids, and proteins. It is also a key source of side effects associated with standard cancer treatments. Antioxidant supplementation, including vitamins A, C, and E, and polyphenols, such as tannic acid, gallic acid, and epigallocatechin gallate (EGCG), can reduce adverse reactions and toxicities related to oxidative stress and have been proven effective through various clinical studies. Despite their strong potential, very few antioxidants are approved in medicine due to several limitations such as low stability, poor water solubility, low bioavailability, and short circulation time in the bloodstream leading to low antioxidant efficiency and longevity.1,2,3
To circumvent limitations associated with antioxidant supplements, antioxidant polymers are considered effective new generation antioxidant therapies.4,6. Polymeric antioxidants have prolonged stability and circulation and can avoid rapid clearance by the immune system, resulting in better bioavailability and antioxidant potential. Furthermore, since polymers can be engineered into several useful forms such as films, gels, solutions, and nanoparticles, antioxidant polymers can be delivered to the site of interest and release encapsulated drugs in a controlled response to inflammatory environments. Antioxidant polymer platforms can be designed to respond to several biological factors such as pH, redox potential, and enzymes, as well as exogenous stimuli such as light, magnetism, ultrasound, and X-rays (Figure 1). As such, antioxidant polymeric platforms serve as transport vehicles for effective drug delivery and seamlessly act as RONS scavengers throughout the therapeutic process.
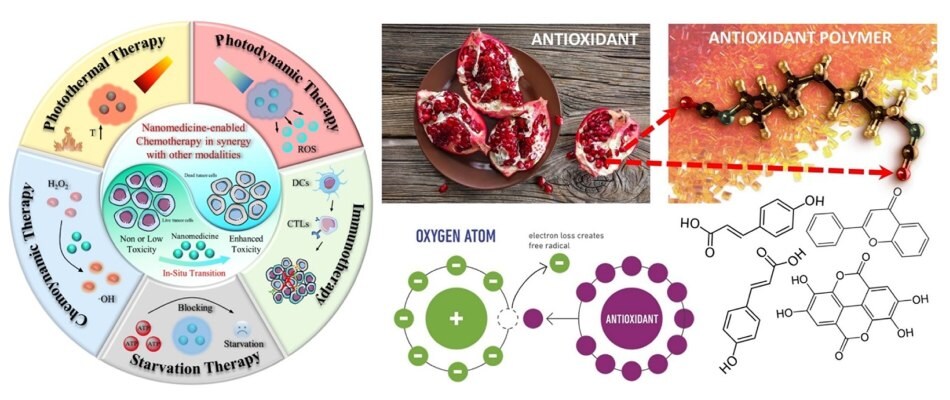
Figure 1.A) A schematic representation of several nanomedicine-enabled chemotherapy routes and related cancer therapies. Reprinted with permission from reference 9, copyright 2022 Springer. B) Antioxidant molecules can be extracted from several different plants, fruits, and even their waste after being processed. They can effectively reduce or eliminate electron-loss related free radical formation in metabolic systems. Reprinted with permission from reference 4, copyright 2021 Multidisciplinary Digital Publishing Institute.
Polymers With Antioxidant Moieties
Historically, polymers with antioxidant moieties were first developed industrially to prevent polymers from oxidative degradation. For instance, polypropylene was rendered resistant against oxidative degradation by attaching cross-linkable antioxidant moieties to the main chain.7 Biotechnology and biomedicine applications have modified biomedical polymers similarly to scavenge ROS and RONS, such as grafting ellagic acid to chitosan.8 Likewise, vanillin, a powerful natural antioxidant (Figure 2A), can crosslink with chitosan and, as such, it increases its mechanical properties and transforms chitosan into an antioxidant polymer.10
In addition, new antioxidant polymers are being synthesized via chemo-enzymatic methods such as biobased linear aliphatic-aromatic oligomers from ferulic acid and trametes versicolor laccase.11 High molecular weight hydroxycinnamate-derived polymers from Symphytum asperum Lepech (a flowering plant) were also demonstrated to be effective antilipoperoxidants (depletes lipid oxidation). This is important because oxidative stress and lipid peroxidation levels can increase during different therapeutic modalities in cancer treatment, contradicting the treatment itself.12,13
In medical sciences, liver-related hepatic ischemia/reperfusion (I/R) injury is strongly associated with H2O2-mediated oxidative stress and is a crucial arbiter of cellular and tissue damage. Therefore, H2O2 is a significant therapeutic target for oxidative stress-associated inflammation. Poly(vanillin oxalate) (PVO) polymer (Figure 2B) generates CO2 through H2O2-triggered oxidation of peroxalate esters and releases vanillin, which exerts antioxidant and anti-inflammatory activities and inhibits inflammation and apoptosis.14 Recently, synthesizing antioxidant polymers from lignin derivatives is also of significant interest.4 Phenols with various chemical structures can be obtained from lignin deconstruction; among them, vanillin and ferulic acid. Various polymers can be synthesized via different chemical modifications and polymerization pathways.
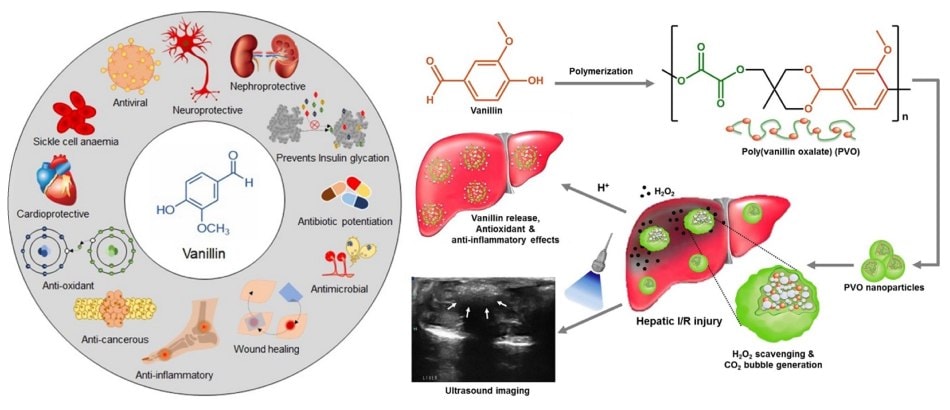
Figure 2.A) The antioxidant molecule, vanillin. This natural extract can easily be polymerized, but as a molecule, it has been shown to display excellent therapeutic properties such as anticancer, antioxidant, anti-inflammatory, neuroprotective, anti-sickling, anti-amyloid aggregation, and inhibition of non-enzymatic glycation, antibacterial, anti-fungal, anti-quorum sensing, antibiotic potentiation, wound healing/tissue engineering, and antiviral. Reprinted with permission from reference 15, copyright 2021 Springer Singapore. B) A schematic diagram illustrating antioxidant PVO polymer with H2O2-triggered echogenic properties and intrinsic antioxidant and anti-inflammatory activities. Treatment with PVO increases the echo intensity for ultrasound imaging and exerts therapeutic effects in H2O2-accompanying ischemia/reperfusion (I/R) injury. Reprinted with permission from reference 14, copyright 2016 Elsevier.
Unlike vehicle-based antioxidant depot approaches, covalently tethering antioxidants to polymers can enable a higher relative antioxidant content by increasing its longevity and presence. The structural incorporation of antioxidant moieties into polymers may offer more flexibility in applying polymer systems for therapeutic use, whereas encapsulation approaches are often limited to particle systems. Among common antioxidant supplements, vitamins A, C, and E are especially interesting for polymerization. These three vitamins are well known as antioxidants, and their low to no toxicity and regulatory approval make them advantageous to alternative new materials. However, many vitamins have very low tolerance for light, heat, and moisture exposure, complicating bioactivity retention during polymerization or grafting.16 Even though their copolymerization is not necessarily straightforward, grafting vitamins to biomedical polymers while retaining a strong antioxidant effect can provide identical antioxidant protection in vivo to the vitamin alone.
Retinoic acid, the most biologically active form of vitamin A, plays an essential role in the induction of cell differentiation and the arrest of cell proliferation. It has been used in stem cell engineering and cancer therapy, particularly in differentiation therapy for acute myelogenous leukemia. However, it has deficient blood circulation levels due to rapid metabolization by the liver. The circulation can be improved by grafting retinoic acid to water-soluble chitosan oligosaccharide to form an antioxidant polymer drug.17 Similarly, an interesting study on vitamin E showed that Trolox, a synthetic antioxidant and water-soluble analog of vitamin E, was polymerized to form an oxidation active polymer as a new class of biomaterial. This antioxidant polymer inhibited intracellular ROS formation and reduced basal ROS levels in monocytes and endothelial cells. It also effectively inhibited direct effects of oxidative stress like protein carbonyl formation, 3-nitrotyrosine levels, and 4-hydroxy-2-trans-nonenal levels in endothelial cells.18
Metal-chelating Intrinsically Antioxidant Polymers
Chelating drugs and chelator metal complexes are needed to prevent, diagnose, and treat cancer. Both cancer and normal cells require essential metal ions such as iron, copper, and zinc for growth and proliferation. Chelators can target the metabolic pathways of cancer cells by controlling proteins involved in regulating these metals. However, excess metal ion uptake by the cells can trigger and catalyze RONs, and special chelators can potentially inhibit the oxidative free radical damage on DNA caused by iron and copper catalytic centers. Specifically, chelating excess iron in the body can effectively fight tumors and side effects of certain cancer therapeutic modalities. To this end, a newly synthesized poly(1,8-octanediol-co-citrate-co-ascorbate) (POCA), a citric-acid based biodegradable elastomer with native and intrinsic antioxidant properties, could be a promising start (Figure 3A). POCA, synthesized from 1,8-octanediol, citric acid, and ascorbic acid, reduced ROS generation in cells and protect cells from oxidative stress-induced cell death (Figure 3B). Importantly, POCA antioxidant properties remained present upon degradation.19
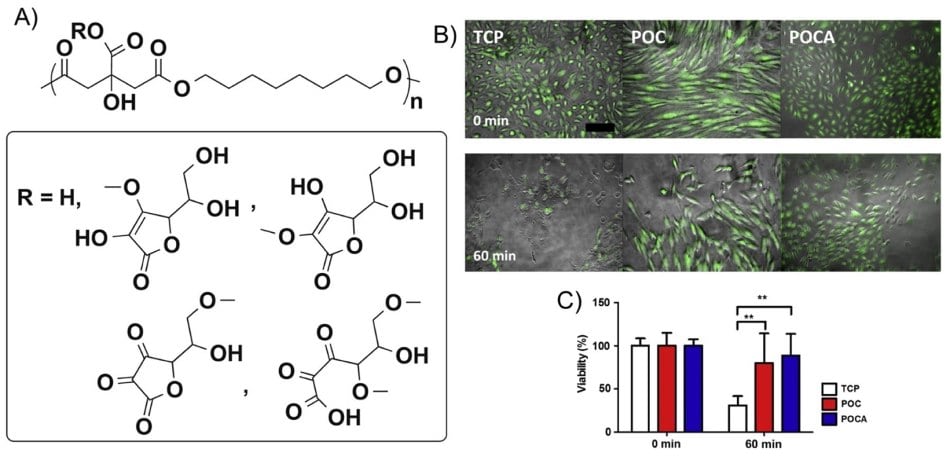
Figure 3.A) Proposed structure and scheme of poly(1,8-octanediol-co-citrate-co-ascorbate) (POCA). In case of R = H, the copolymer is poly(1,8-octanediol-co-citrate) (POC). B) Human umbilical vein endothelial cells (HUVECs) cultured on top of POC and POCA showed prolonged survival upon menadione stimulation for 60 min. POC and POCA provided protection from excessive ROS-induced cell death. Combined overlap image of contrast light microscopy with fluorescent image shown. Green indicates calcein AM stain for live cells. Bar = 200 μm. C) Quantification of percentage of calcein AM positive cells after 120 min relative to unchallenged cells. N = 4, Mean ± S.D., **p < 0.01. TCP stands for tissue culture plate. Reprinted with permission from reference 20, copyright 2014 American Chemical Society.
Another very effective antioxidant polymer with metal chelating capability was demonstrated as a biodegradable, thermoresponsive gel with intrinsic antioxidant properties suitable for the delivery of therapeutics. Citric acid, poly(ethylene glycol) (PEG), and poly-N-isopropylacrylamide (PNIPAAm) were copolymerized by sequential polycondensation and radical polymerization to produce poly(polyethylene glycol citrate-co-N-isopropylacrylamide) (PPCN).20 PPCN exhibited free radical scavenging activity, chelated iron ions, and inhibited lipid peroxidation while enabling chemokine delivery in a controlled manner (Figure 4A). Additionally, PPCN immediately formed a macroscopic gel in situ when injected into rat subcutaneous tissue, facilitated new connective tissue formation with a minimal foreign body response, and was resorbed by the body at 30 days post-implantation (Figures 4B–D).

Figure 4.A) Poly(polyethylene glycol citrate-co-N-isopropylacrylamide) (PPCN) polymer gel structure. The PPCN gel has a lower critical solution temperature (LCST) of 26 °C and exhibits intrinsic antioxidant properties based on its ability to scavenge free radicals, chelate metal ions, and inhibit lipid peroxidation. Subcutaneous injection of 150 µL of PPCN (100 mg/mL, pH = 7.4) in female Sprague Dawley rats. Black arrows denote gel location B) directly after injection on the back of rat, a palpable lump was formed C) PPCN gel after 3 days D) frozen section of PPCN gel in tissue harvested at 30 days. Most of the gel has been replaced by new tissue. Reprinted with permission from reference 20, copyright 2014 American Chemical Society.
Desferrioxamine (DFO), deferiprone (DFP), desferasirox (DFX), and ethylenediaminetetraacetic acid (EDTA) are currently the only FDA-approved metal-chelating compounds for specific removal of metals from the metabolism. Despite FDA approval, these compounds have limited bioavailability, insufficient selectivity, neurotoxicity, and other conflicting results on therapeutic performance, particularly regarding EDTA. A new DFO polymer system was developed by glycidol ring opening and aldehyde reactions with sodium periodate. The polymeric iron chelator was synthesized by conjugating DFO into hyperbranched polyglycerol (HPG) and resulted in improved circulation lifetimes and removal of excess iron in vivo. Additionally, the polymer effectively regulated plasma ferritin levels, which could potentially benefit patients suffering from transfusion iron overload.21 A range of iron binding dendrimers terminated with hexadentate ligands derived from hydroxypyridinone, hydroxypyranone, and catechol moieties was synthesized to serve as clinically useful iron (III)-selective chelator polymers. The iron chelating abilities of these and similar polymers should be considered, for instance, for potential iron overload after tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk neuroblastoma.22
One unique application of metal-chelating polymers is in highly specific radiolabeling of monoclonal antibodies (mAbs) and their effect on the tumor and normal tissue uptake. Radioimmunotherapy (RIT) employs mAbs complexed into radionuclides that emit α-particles, β-particles, or Auger electrons for systemic radiation treatment of cancer. However, delivery and penetration barriers for radiolabeled mAbs still exist for solid tumors. One potential approach to overcome this hurdle is to increase the proportion of antibody molecules carrying a radionuclide within tumor cells or masses. Most commonly, radiolabeling is performed by conjugating chelators to the mAbs such as diethylenetriaminepentaacetic acid (DTPA) or tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to form a complex with radiometals, yet with very low substitution/chelation yields. A new metal-chelating polymer, based on a polyglutamide (PGlu) backbone with grafted DOTA and polyethylene glycol (PEG), showed very effective RIT treatment of different-sized tumor deposits.23,24
Antioxidant Polymer Nanotherapies
As previously discussed, instead of encapsulating the antioxidants into polymer gels or nanoparticles, an alternative method offering promising new treatments is conjugating the drug or antioxidant directly to a degradable or non-degradable polymer. This approach is driven by the growing market of pharmaceutically active yet structurally fragile pro-drugs, where any selected functional group of the drug molecule is modified to protect from deactivation and allow for conversion back to the active form upon administration. Many medical conditions can now be treated with emerging nanotherapeutics such as thrombus, which is associated with excess formation of harmful H2O2.25 Since most small molecule antioxidants possess one or more hydroxy or thiol groups, they can be functionalized and formulated into polyester, polyanhydride, or poly(β-amino ester) polymeric structures. For instance, poly(beta-amino ester) (PBAE) chemistry was employed to conjugate quercetin and curcumin into crosslinked polymer films, microparticles, nanoparticles, or nanogel systems.26 Briefly, the polymer was synthesized by reacting acryloyl chloride and triethylamine with curcumin or quercetin, forming an intermediary acrylic. The formed acrylic reacted with PEG diacrylate (PEG400DA), a primary diamine such as 4,7,10-Trioxa-1,13-tridecane diamine (TTD), 2,20 (ethylenedioxy)-bis-ethylamine (EDBE), and hexame-thyldiamine (HMD) to form a crosslinked polymer network. The nanogels, 50 to 800 nm in size, provided uniform release over 24–36 hours by bulk hydrolytic degradation. Both curcumin and quercetin conjugated systems demonstrated protection against induced oxidative stress in endothelial cells. The curcumin PBAE nanogels were explicitly studied for their action on mitochondrial oxidative stress, a significant source of ROS and RONS production, and were found to be more effective at interrupting ROS production than free curcumin.
Enhanced bioactive antioxidant formulations are critical for the treatment of inflammatory diseases as well, such as atherosclerosis. A hallmark of early atherosclerosis is the oxidized low-density lipoprotein (oxLDL) uptake by macrophages, which results in foam cells and plaque formation in the arterial wall. A new class of nanoparticles with antioxidant polymer cores and shells comprising scavenger receptor targeting amphiphilic macromolecules based on diglycolic acid linked ferulic acid- poly(anhydride-ester) were shown to counteract the uptake of high levels of oxLDL and regulate ROS in human monocyte-derived macrophages (HMDMs).27 A recent relevant study demonstrated a novel class of antioxidant polymer nanoparticles attenuated ultraviolet B radiation-induced skin inflammatory disorders in Kud:Hr- hairless mice. These nanoparticles were administered orally, as the technology is designated as oral nanotherapeutics.28
Additionally, antioxidant polymer nanoparticles made from methoxy-poly(ethylene glycol)-b-poly(chloromethylstyrene) (MeO-PEG-b-PCMS) provided an effective anti-aging strategy for the protection of the skin against ROS and the reduction of the skin-damaging effects of prolonged UV exposure. Briefly, MeO-PEG-b-PCMS was synthesized by the radical polymerization of chloromethylstyrene (CMS) using methoxy-poly(ethylene glycol)-hydroxyl with a grafted chain transfer agent. Subsequently, the chloromethyl groups were converted to a nitroxide radical moiety by an amination reaction with the NH2-TEMPO radical (Figure 5A). This amphiphilic block copolymer was used to synthesize orally administered nanoparticles via hydrophobic interactions of the active NH2-TEMPO segments. Upon dissociation at low pH, the redox polymer was released and circulated in the blood to provide protection against ROS.
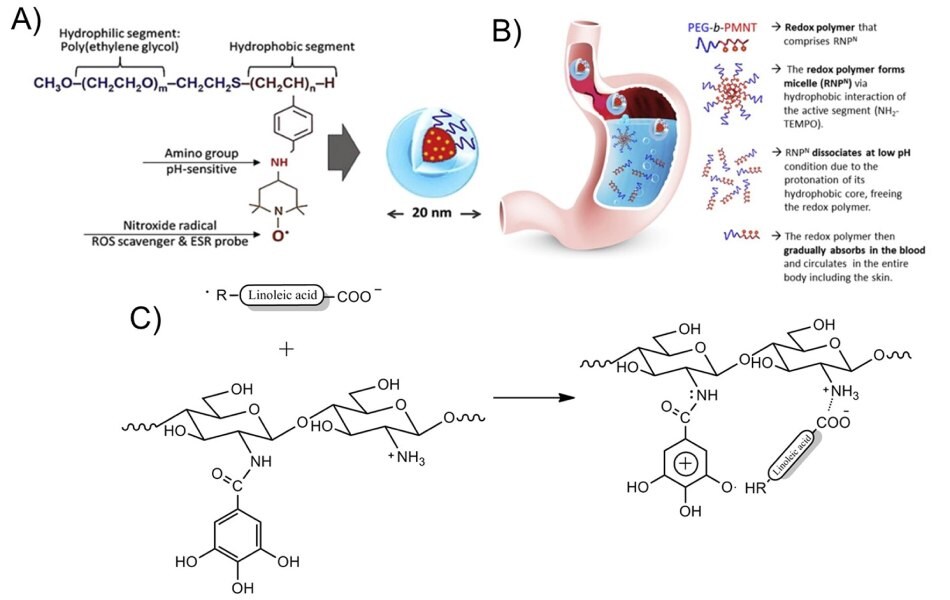
Figure 5.Design of oral nanoparticles, their delivery, and absorption in the body, including the skin. A) Chemical structure of the pH-sensitive polymer nanoparticles. B) Graphical illustration of the oral delivery of the antioxidant polymer nanoparticles and their absorption into the body and the skin area. The unique characteristics of the nanoparticles allow the gradual absorption into the bloodstream after oral administration, keeping their bioavailability high for their effective ROS scavenging effect. Reprinted with permission from reference 28, copyright 2017 Elsevier. C) Proposed interaction between gallic acid–chitosan and free linoleic acid radicals. Reprinted with permission from reference 29, copyright 2014 American Chemical Society.
Another promising edible antioxidant polymer that can be transformed into nanoparticles is gallic acid grafted chitosan which exhibited stronger antioxidant potential and adjustable rheological properties, than pure gallic acid.29 Similarly, conjugating chitosan with chlorogenic acid also formed robust antioxidant polymer systems against lipid peroxidation. The copolymer demonstrated better antioxidant potential, and its capacity to inhibit β-carotene-linoleic acid bleaching and lipid peroxidation was stronger than chlorogenic acid alone.
Epigallocatechin-3-gallate (EGCG) is the most active catechin of tea polyphenols and accounts for 50–80% of the total catechin present in green tea. Potential health-promoting properties of EGCG include strong antioxidant activity, cancer chemoprevention, radioprotection, cardiovascular health improvement, and anti-obesity capabilities. EGCG was reported to be approximately 10 times as effective as β-carotene and L-ascorbate in alkyl peroxyl radical scavenging.30 However, EGCG's bioactivity is limited by its low oral bioavailability and poor stability. EGCG is highly soluble in water; however, it can be easily oxidized in the aqueous phase. It can be grafted into proper polymer backbones to circumvent these limitations and serve as a robust theranostics polymer. To this end, β-Lactoglobulin (BLG)–chlorogenic acid (CA) conjugates with controlled grafted amounts of EGCG were generated by a free radical-induced grafting method. The resulting nanoparticles had an average size of 100 nm and encapsulated up to 75% EGCG. Their use and functionality in cancer therapeutics have yet to be researched. However, they are expected to produce promising results that could pave the way for synthesizing other functional β-Lacto globulin-based polymer nanoparticles.
Finally, we would like to stress the importance of designing antioxidant polymers from metalloproteins (a protein that contains a metal ion cofactor) such as polymer/hemoglobin assemblies.31 This approach is not to encapsulate hemoglobin like a drug but to form polymeric hemoglobin nanostructures that can be used to treat health problems effectively related to hypoxia, for instance. Promising strides have already been made. For example, polymeric nanoparticles obtained from hemoglobin conjugated with antioxidant enzymes via dicarboxymethylated poly(ethylene glycol) (PEG) linkers and ring opening polymerization (Figure 6) have shown a beneficial effect in protecting pancreatic beta cells from severe hypoxia at transplantation sites.32 Similarly, polyhemoglobin-superoxide dismutase-catalase was designed to function as an oxygen carrier with antioxidant properties. The polymer was prepared by crosslinking hemoglobin with superoxide dismutase and catalase with glutaraldehyde. This hemoglobin polymer decreased the formation of oxygen radicals compared to hemoglobin alone in a rat intestinal ischemia-reperfusion model.33 A novel thermo-responsive hemoglobin-polymer conjugate was also reported. The polymer was synthesized by reacting the lysine amino groups of hemoglobin and carboxyl groups of a copolymer, poly(N-isopropylacrylamide) grafted carboxylated dextran, via single electron transfer living radical polymerization. Upon heating above LCST, the conjugate could form isolated uniform spherical nanoparticles.34
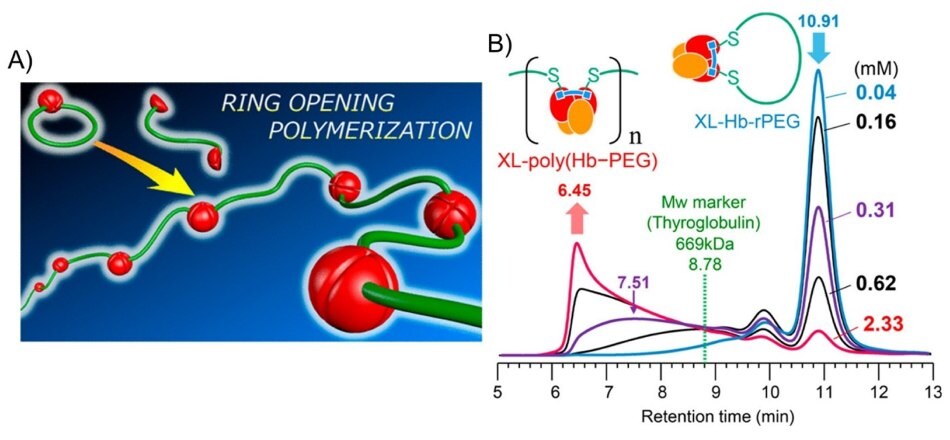
Figure 6.A) A new class of hemoglobin polymer by ring-opening polymerization. The polymer, poly(Hb–PEG), is synthesized by reacting native hemoglobin with bifunctional maleimide-PEG. The ring opening polymerization occurs by exchanging αβ subunits intermolecularly to produce poly(Hb–PEG). B) Size-exclusion chromatography (SEC) profiles obtained for products of reacting Hb-PEG monomer with bis(3,5-dibromosalicyl) fumarate (DBBF) at different monomer concentrations of 0.04, 0.16, 0.31, 0.62, and 2.33 mM. Reprinted with permission from reference 35, copyright 2019 American Chemical Society.
Future Perspectives
Due to ongoing elucidation of the exact role of oxidative stress in a plethora of pathologies, recent polymer science research has led to a remarkable increase in studies related to oxidative stress-lowering therapies. The main aim of this article was to demonstrate that polymeric antioxidants could be the next generation “antioxidants” in theranostics, owing to their potent antioxidant properties and increased stability in aqueous systems compared to many pure phenolic molecules. To overcome the challenges and limitations of direct, systemic administration of antioxidants, such as instability, absorption limitations, low solubility, diminished bioavailability, and the limited permeability through the cell membranes of many antioxidant molecules, polymer-based antioxidant therapies are now actively investigated. To be genuinely effective in biological applications, these polymers must be synthesized using benign methods. A deeper understanding of factors affecting antioxidant activity would provide an opportunity for the design of versatile, high-performing polymers with enhanced antioxidant activity. Since naturally occurring phenolic compounds have superior ROS scavenging profiles, these molecules can serve as excellent starting materials for synthesizing polymeric antioxidants. Enzyme catalyzed polymerization should be more studied as it enables the synthesis of polymeric phenols under environmentally friendly conditions.
Many biomedical polymers with known pro-oxidant degradation products such as polycaprolactone, polylactic acid, or polyglycolic acid should be engineered to be neutral or even antioxidant by copolymerization with antioxidants. This will enable a gradual shift from encapsulation approaches to the structural incorporation of antioxidants into the polymer system. Yet, most reports on cell viability data do not necessarily compare the measured upper limits for cell survival with the local concentrations needed for effective oxidative stress modulation. This makes it quite problematic to judge whether antioxidant polymer technologies qualify for addressing particular pathologies. At the same time, our understanding of how natural antioxidants function has improved. For instance, recent studies on the structure and antioxidant properties confirm that the antioxidant properties of flavonoids increase due to the presence of the hydroxyl group in the C3 position and the catechol moiety. In the case of kaempferol and quercetin, the 3-OH hydroxyl group is the most involved in forming a radical structure. The delocalization of electronic charge because of ligand complexing with metals of high ionic potential (Fe (III), Ln (III) Y (III)) can lead to increasing the antioxidant properties, according to our preliminary studies, even by up to 10 times. However, the positive or negative roles of these metals during theranostics applications are to be evaluated very carefully.
New polymer synthesis reports are encouraging and promising, such as electroactive and biocompatible tetra(aniline)-based terpolymers with tunable intrinsic antioxidant properties in vivo. These polymers are obtained from non-biodegradable or inedible compounds such as tetra(aniline), fumaryl chloride, and polyethylene glycol. However, upon polymerization transform into biodegradable and non-toxic antioxidant polymers. Cationic 1,2,3-triazole functionalized starch derivatives are promising new systems that still lack extensive medical theranostics studies. As such, future studies should take into account (i) analyzing the impact of prolonged exposure to various level concentrations of polymer (nano) antioxidants; (ii) assessing the potential risks during fabrication, manipulation, and storage; (iii) simplifying the fabrication processes for the manufacture of highly potent systems; (iv) enhancing the reproducibility, biocompatibility, reliability, robustness, and stability of polymer (nano) antioxidants in biological microenvironments; and (v) designing microreactors that can effectively control the parameters of reaction synthesis.
Polymeric antioxidants have exhibited high potency for oxidative stress depletion with high cellular antioxidant potential, specificity, and targeted delivery. However, to achieve significant clinical benefits, a thorough understanding of the detailed antioxidative mechanisms of these new polymers necessitates extensive cross-collaboration of experts from various fields, including materials science, chemistry, and physical and biomedical fields. In-depth mechanistic studies of oxidative stress in vivo will be helpful in revolutionizing the polymer antioxidant therapeutic field.
References
To continue reading please sign in or create an account.
Don't Have An Account?