In Vitro T Cell Killing Assay Protocol using Millicell® Microwell Plates
T Cell Killing Assays
T cells, a vital component of the human immune system, are critical to combating cancerous tumors or infections. Today, new immunotherapies are being developed that activate T cells and increase effector function, suppress inhibitory mechanisms, or move T cells in proximity to their target cells. T cell killing assays are used to determine new drug potency, the immune modulation of novel immunotherapies, and for selecting promising candidates for drug studies using animal models.
With the recent developments in 3D cell culture and organoid technology, multiple T cell killing assays have been established to test T cell functionality and response to immunotherapies in vitro. Here, our robust and scalable T cell killing assay evaluates the killing capacity of patient-derived tumor-infiltrating lymphocytes (TIL) in combination with immunomodulators to autologous human colorectal cancer tumor spheroids using Millicell® Microwell 96-well plates. The assay enables patient-specific assays due to the use of patient-derived tumor spheroids and TILs.
What are Millicell® Microwell Plates?
Current T cell killing assay systems have limited throughput, are often difficult to handle, and can result in low reproducibility. This makes them difficult to scale up for high throughput or screening applications. Millicell® Microwell 96-well plates are a ready-to-use platform for scalable and reproducible organoid culture.
The wells in the plates contain a ready to use polyethylene glycol (PEG) hydrogel solution, and no external solid extracellular matrix (ECM) is required. The PEG solution within the wells is biocompatible and allows for the efficient aggregation of stem cells. It is also water based, which promotes diffraction-less imaging.
Since spheroids or organoids formed in the wells are in a single focal plane, the Millicell® Microwell plates are ideal for automation workflows and imaging. To combat microtissue loss, Millicell® Microwell plates also have a media exchange port for easy media exchange without disturbing the microtissues. This standardized and homogeneous organoid culture is ideal for the easy implementation of 3D cell culture research applications.
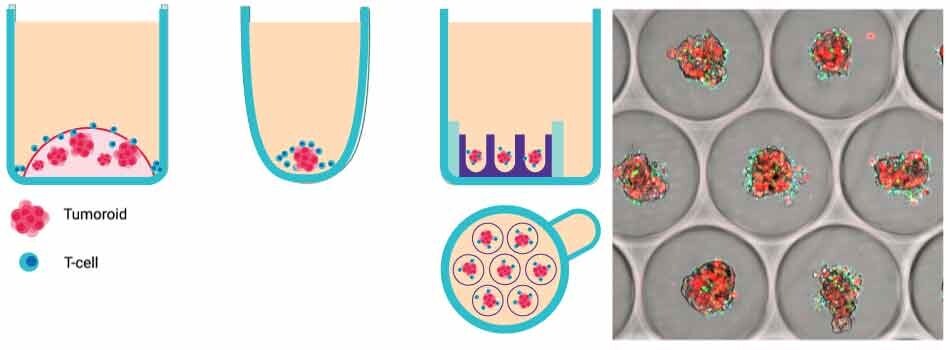
Figure 1. A. Millicell® Microwell plate T cell killing assay overview. Schematic representation of in vitro T cell killing assay systems. From left to right: external ECM-embedding (e.g., Matrigel), non-adherent surface, Millicell® Microwell plate. B. Brightfield image of tumor spheroids formed using the Millicell® Microwell 96-well plates.
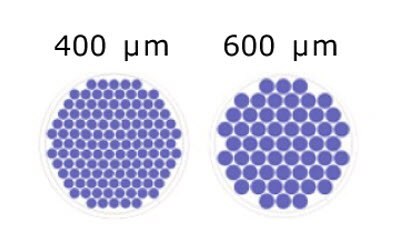
Figure 2.Schematic representation of the number of microwells/well based on microwell size for Millicell® Microwell 96-well plates.
T Cell Killing Assay Protocol
Using this protocol, up to tumor spheroids were formed in a single well and co-cultured with TILs. The T cell killing potential used semi-automated image analysis and was performed in situ.
- Seed human colorectal cancer spheroid forming cell line into the microcavities using the O-ring in the 600μm Millicell® Microwell 96-well plate.
- Add culture media through the media exchange port. Note: if an ECM is required, it can be diluted into the media and added in this step. Cells will compact in the suspension-like culture.
- Let the tumor spheroids grow and fully develop. Replace media through the media exchange port after 4-6 days.
- Add TILs until the desired effector to target (E:T) ratios are reached. Note: this assay was performed in the presence or absence of immunomodulators IL2, CD3 (IL2 + -CD3/CD28), and PD1 (IL2 + -CD3/CD28 + -PD1/CTLA4).
- Monitor growth over time using cell trackers1. For this assay, tumor cells, T cells, and dead cells were labeled with far-red tracer (blue, CellTrace™ Far Red Cell Proliferation Kit; Molecular Probes), CFSE (green), and propidium iodide (red), respectively.
For T cell staining:
- Resuspend cells in CFSE staining solution (1:200 in assay buffer followed by a dilution 1:2 in warm PBS) and incubate at 37˚C for 20 minutes.
- Add equal volume of media with 10% FBS and incubate for 10 minutes.
- Spin down at 200xg for 5 minutes.
- Resuspend cells in media at the desired concentration; such as 10M cells/ml.
For organoid staining:
- Resuspend cells in 1ml warm PBS with trace (1:1000) and incubate at 37˚C for 20 minutes.
- Add equal amounts of media (DMEM + 10% FBS) and incubate for 5 mins at 37˚C.
- Spin down at 200xg for 5 minutes.
- Resuspend cells in 1 ml of media.
- Add propidium iodide stain to assess T cell killing. Dilution of propidium iodide will depend on the supplier recommendations.
- Perform semi-automated image analysis to quantify cell death, tumor size, and TIL migration.
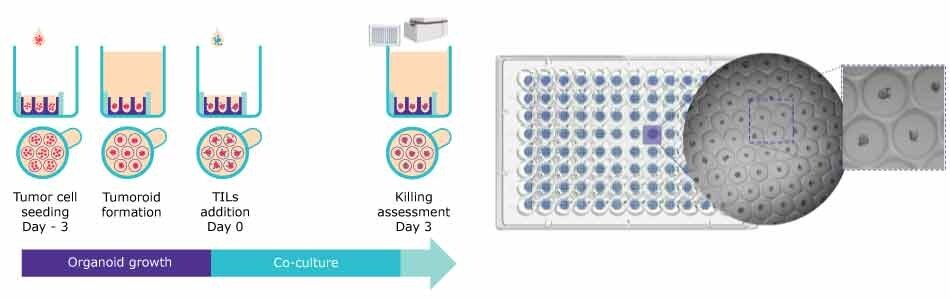
Figure 3. T cell killing protocol using Millicell® Microwell 96-well plates. A. Assay workflow for the organoid growth and formation and assessing the co-culture with TILs. B. Representative Brightfield image of human colorectal tumor spheroids.
Millicell® Microwell T Cell Killing Results
The Millicell® Microwell 96-well plates allowed for increased cell-cell contact and the controlled co-culture of tumor spheroids in direct contact with the T cells, similar to what occurs in vivo. Because multiple uniform tumor spheroids can be evaluated in a single well, there is a significant increase in the number of datapoints per well.
Our assay shows that the selected patient sample had autonomous T cell activation and increased cytotoxic response in the presence of immune checkpoint inhibitors. At day 3 after spheroid formation, the total area of the tumor spheroid decreased in the presence of the immunomodulators (Figure 4). This indicates that the addition of immunomodulators led to tumor death and shrinkage. Higher effector to target ratios result in more efficient killing. This was observed by an increased propidium iodide signal through fluorescent imaging.

Figure 4.Patient-derived colorectal tumor spheroids and autologous TILs co-culture assessment. A. Brightfield microscopy images of tumor cells (blue), with T cells 10:1 E:T ratio (green) and dead cells (red). Scale bar 500μm. B. Quantification of propidium iodide staining at day 3; C. Quantification of tumor spheroid area at day 3.
This assay was validated using the Pmel-1 transgenic mouse model. Melanoma tumor spheroids were disrupted in the presence of T cells but were able to grow steadily in the absence of T-cells. As seen in the in vitro T cell killing assay, high E:T ratios lead to more efficient tumor killing.

Figure 5.Pmel-1 mouse model T cell killing assay. A. Brightfield imaging of B16-F10 melanoma tumor spheroids co-cultured with targeting T cells at 10:1 E:T. Scale bar 500μm. B. Quantification of propidium iodide intensity at day 2.
References
To continue reading please sign in or create an account.
Don't Have An Account?