Small Molecule Contract Testing, Development, & Manufacturing Services

Small Molecule synthesis, from preclinical to commercial quantities
With Millipore® CTDMO Services we support small molecule API synthesis projects from milligram to commercial quantities. We are the partner to address the most difficult challenges, whether the target is difficult to handle, synthesize, or source. With a true consultative and partnered approach, our services reduce complexity and minimize program risk in a cost-effective manner.
Clients can speed up drug development by partnering with us on any of the following types of programs: HPAPIs, APIs, Next-Generation Conjugates, Linker-Payloads, PEGs, or Targeted Protein Degradation.
Our Track Record
#1
World's largest single-digit nanogram OEL CTDMO provider
30+
Years of experience in small molecule development and production
7
Small molecule modality targets
70,000
Square foot HPAPI expansion in Verona, WI
2
New large scale API production lines opening in Europe
Your Drug Journey

Preclinical
From conceptualization to the first GMP batch, our project teams work in close collaboration with our client’s scientists to ensure their vision becomes a reality.

Clinical
Our quality and regulatory experts are available to provide guidance on a phase appropriate and efficient approach throughout the various clinical stages.

Commercial
As a program approaches commercialization, we offer consultation and implementation of new technologies and efficiencies to ensure the drug is competitive in the marketplace upon approval.
Our Offering
Active Pharmaceutical Ingredients (APIS)
With decades of experience across a variety of chemistries and therapeutic indications, we are the team for custom API synthesis. From early development to commercial supply, our global network of development and manufacturing facilities is ready to support our clients’ programs. With Millipore® CTDMO Services we offer:
- Global geographic reach, with development and manufacturing resources in both North America and Europe
- Batch size scalability, from gram scale up to metric ton quantities
- Secondary supply capabilities within our own organization for business continuity planning and risk reduction
- Robust support services, including large scale hydrogenation, cryogenic reactions, and non-GMP continuous flow manufacturing
- Strong quality and regulatory support from pre-clinical to commercial programs
Highly Potent Active Pharmaceutical Ingredients (HPAPIS)
With over 30 years of experience in the production of highly potent active pharmaceutical ingredients (HPAPIs), we are an industry leader with the expertise needed to tackle the unique handling challenges of these therapeutic compounds. With Millipore® CTDMO Services we offer:
- Handling capabilities down to single digit nanogram containment
- Industrial hygiene certified facilities with SafeBridge® Industry Leadership in HPAPI handling certification
- A comprehensive portfolio of payload and advanced payload intermediate products to accelerate drug development efforts
- Strong quality systems and regulatory support from pre-clinical to commercial programs
- Range of scale capabilities, industry leader in low as well as high volume production of potent APIs
Activated Polyethylene Glycol(PEGS)
With decades of expertise in activated and functionalized PEGs, we offer the technical, quality, and regulatory support needed to maximize product efficiency through optimized release kinetics and targeted drug delivery. With Millipore® CTDMO Services we offer:
- Tailored development approach to meet our clients’ needs. Support for both polydisperse and monodisperse PEGs, linear to branched, and all varieties of functionalization.
- Advanced analytical expertise to characterize and quantify PEG
- Strong quality systems and regulatory support from pre-clinical to commercial programs
- Global network of manufacturing facilities to supply large-scale commercial demand
Next-Generation Conjugates
As industry experts in development and production of linker-payloads and PEGs as well as ADC bioconjugation, we also offer comprehensive support for Next-Generation conjugate programs. With Millipore® CTDMO Services we offer:
- Tailored development approach to meet client needs across several prominent Next-Generation conjugate modalities
- A comprehensive portfolio of payload and advanced payload intermediate products to accelerate drug development efforts
- Batch size scalability to support progression through the clinic
- Strong quality systems and regulatory support from pre-clinical to commercial programs
Targeted Protein Degradation
As our clients prepare for entry into the clinic, we are here to support in the pursuit of drugging the “undruggable” targets. With a true consultative partnership approach, our experts have the flexibility to provide these unique materials regardless of clinical phase. With Millipore® CTDMO Services we offer:
- Decades of experience with complex synthesis routes, involving numerous synthetic steps
- Phase appropriate approach to develop process and analytical with clinical speed and commercial readiness in mind
- Dedicated project management support for a streamlined experience
- Batch size scalability to support progression through the clinic
- Strong quality systems and regulatory support as programs progress
Linker Payloads
With over 20 years of experience in linker-payload technology, we have a long history with this modality, partnering with most early ADC linker-payload pioneers. Our small molecule development scientists partner with clients to provide technical expertise needed to develop synthetic routes for the most challenging of molecules. With Millipore® CTDMO Services we offer:
- Handling capabilities down to single digit nanogram containment
- Industrial hygiene certified facilities with SafeBridge® Industry Leadership in HPAPI handling certification
- A comprehensive portfolio of payload and advanced payload intermediate products to accelerate drug development efforts
- Strong quality and regulatory support from pre-clinical to commercial programs
- Range of scale capabilities, industry leader in low as well as high volume production of potent APIs
- Seamless integration with downstream ADC/bioconjugation service offer
Drug Development Acceleration Products
Advance your drug development program with our product lines of payloads and advanced payload intermediates. These products offer value by reducing your linker-payload development costs, streamlining your supply chain, and accelerating your synthesis process – speeding up your journey to the clinic. With Millipore CTDMO Services we offer:
- Advanced Intermediates to simplify custom payload synthesis: MAYCore (Advanced Maytansine Intermediate), DOLCore (Advanced Dolastatin Intermediate), and PBDCore (Advanced PBD Intermediate)
- Payloads to support drug conjugate programs: DM1, Exatecan, MMAE
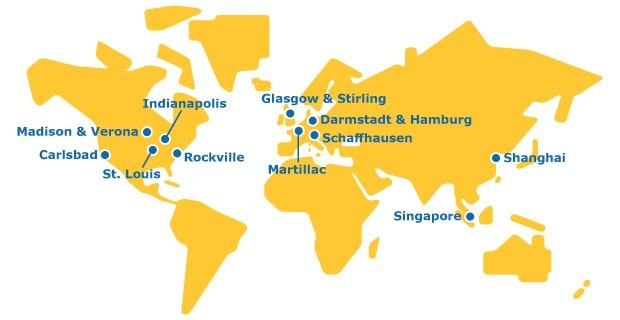
A Global Footprint
We are a single organization with a global network to deliver CDMO services across all stages of the molecule value chain.

Commercialization for large scale API production and portfolio lipids. Continued investment to expand our global network of API production facilities offers immediate development and manufacturing capacity..

Development, manufacturing and launch site for lipids, functionalized polyethylene glycols (PEGs), and APIs.
Our API and HPAPI manufacturing sites are SafeBridge certified. Continued investment and expansion in these facilities build upon our decades of high potency expertise and makes us one of the largest single-digit nanogram OEL CTDMO providers in the world.
Our ADC & Bioconjugation Center of Excellence offers ADC and Bioconjugation capabilities from pre-clinical development to commercial manufacturing.
Related Technical Resources
- Benefits of Monodisperse Activated PEGs in ADC Development
In this white paper, we explore monodisperse polyethylene glycols help overcome hydrophobicity challenges.
To continue reading please sign in or create an account.
Don't Have An Account?

