Keep a close eye on quality and safety
With our excipients for ophthalmic drug formulation
EXCIPIENTS FOR OPHTHALMIC DRUG FORMULATION
Keep a close eye on quality and safety
Ophthalmic drugs pose special formulation challenges to manufacturers. In general, choosing the right processes and materials is important to ensure the required quality, drug tolerability, and patient adherence. Formulators must pay attention to tonicity, pH, stability, and viscosity, as well as sterility and microbiological purity, which has to be assured throughout the time of use. The final ophthalmic formulation must remain sterile, microbially pure, and stable throughout shelf life.
Finding the right excipient that supports these needs is key.
Our large product portfolio covers all excipient groups required for ophthalmic formulation, such as viscosity enhancers, surfactants, buffers and pH regulation agents, isotonicity adjusting agents as well as antioxidants and preservatives – all in the right quality.
Popular Product Categories
Mitigate risk during formulation development
For an ophthalmic formulation to succeed, making sure all of its properties are in line with regulatory guidelines right from the start is essential.
We are aware that developing formulations to treat ocular diseases is challenging and that requirements for the final product are high. To support full supply chain transparency and risk mitigation, we not only provide excipients in the highest quality but also in-depth, consistent documentation in a centralized location, so you can access the information you need when you need it. And when it comes to regulatory expertise, our experts are always here to support you.
What we offer:
- A vast portfolio of top-quality raw materials for all dosage forms in line with ophthalmic industry standards and needs
- Seamless service to support your process from formulation to final fill
- Special capabilities and services such as customized packaging available on request
- Comprehensive ready-to-use Emprove® documentation
Our Emprove® Program, along with the Emprove® Expert portfolio range, combines comprehensive documentation with excellent service, to support risk assessment and qualification for your ophthalmic process.
Preservatives to prevent microbial growth
We offer a broad portfolio of preservatives suitable for multiple administration routes and dosage forms. And to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes, we offer comprehensive documentation as part of our Emprove® Program.
- Comprehensive portfolio of products
- Multi-compendial
- Emprove® Program and documentation
Trehalose for treatment of dry eye syndrome
The disaccharide trehalose is widely used in a variety of pharmaceutical formulations as a bioprotectant, osmoprotectant and stabilizer. In ophthalmics, it is employed in the treatment of dry eye syndrome.
Our product Trehalose dihydrate Emprove® Expert is low in endotoxins and bioburden, making it the right choice for your high-risk applications.
- Multi-compendial, including ChP
- China registration dossier submitted
- Low endotoxin limit (≤ 0.3 I.U./g)
- High-quality grade in a market with limited number of suppliers
- Emprove® Program and documentation
High-quality APIs as alternatives to antibiotics
Our active pharmaceutical ingredients (APIs) are suitable for ophthalmic formulations for eye diseases (e.g. for inflammation, cataract and dry eye syndrome) and can be used as alternatives to antibiotics. Backed by industry-leading production know-how and comprehensive regulatory support, our range of high-quality APIs provides you with all the information and support you need for your ophthalmic product.
- High-quality products
- Manufactured under cGMP ICH Q7 guideline
- CEP and US-DMF available
- Dedicated regulatory support worldwide throughout the entire registration process and an excellent compliance track record
- Emprove® API information package available for your risk assessment and qualification
The Emprove® Program - Your fast track through regulatory challenges
To help you optimize your process, our Emprove® Program provides comprehensive and thorough documentation. It not only covers the latest regulatory requirements, but also anticipates industry expectations not yet covered by regulation. The Emprove® Program is organized into three different types of dossiers. Every dossier supports you throughout different stages of your operations: qualification, risk assessment, and optimization – so you can speed your way through the regulatory maze.
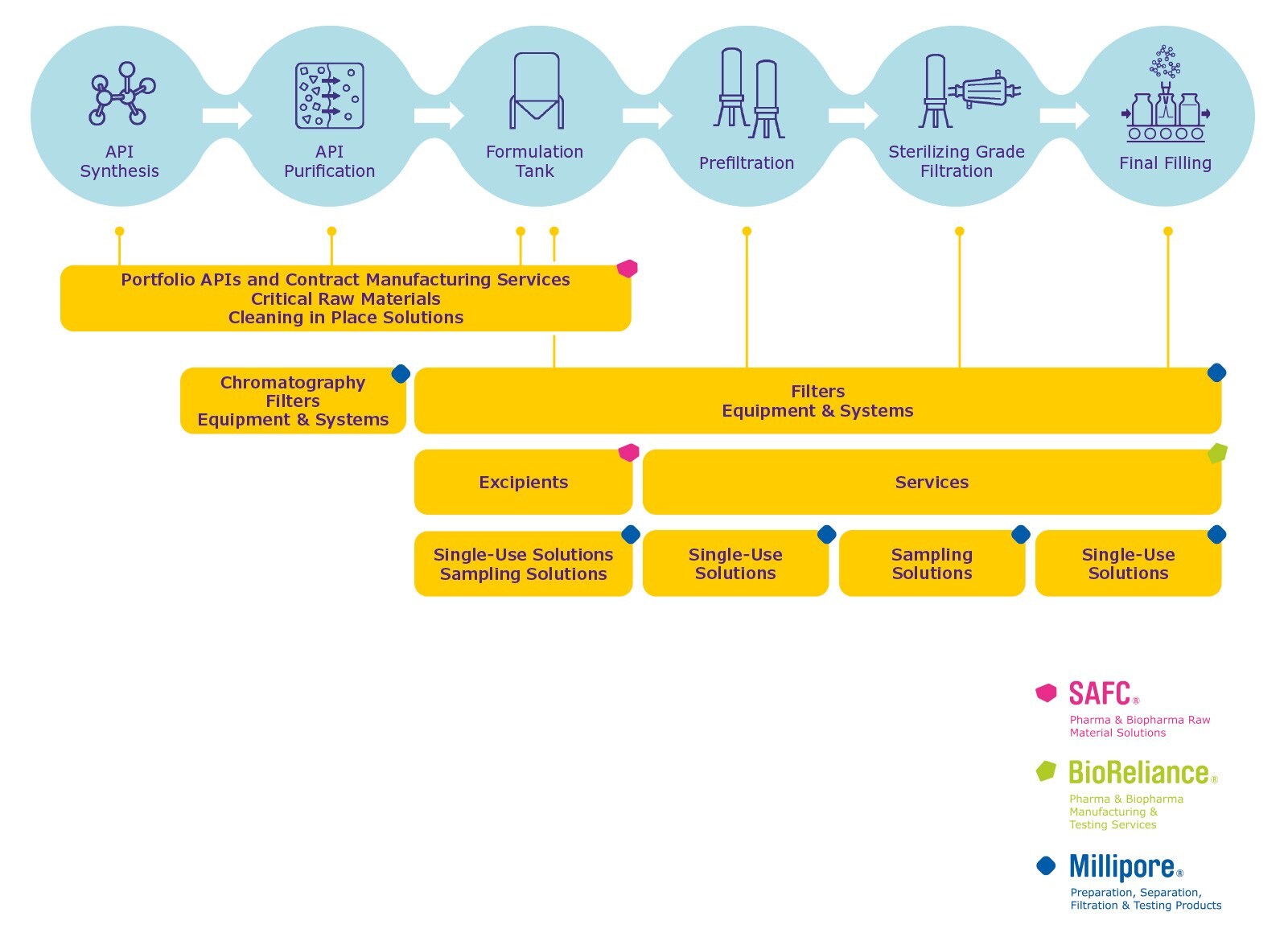
Ophthalmics Process
When designing a manufacturing process for ophthalmic products and selecting a supplier, it is important to consider the full breadth of needs from API synthesis to final filling:
- An integrated portfolio and contract manufacturing capabilities in APIs, critical raw materials (Chemiflex™), intermediates, solvents, excipients and other reagents (incl. cleaning in place) to ensure the required performance, quality and regulatory compliance in every step of your process
- Services to support development, compatibility and integrity testing
- A comprehensive range of chromatography solutions, filters and hardware for your purification steps
- Final filters to ensure bioburden and particulate control and sterility in aseptic processes
- Regulatory expertise and comprehensive documentation to meet stringent regulatory requirements
Related Product Resources
- Technical article: Overcoming challenges in ophthalmic formulations through polymer selection
Read about the careful consideration of polymer type used and how they affect sterilization and final formulation
- Technical article: Use of polyvinyl alcohol (PVA) to overcome challenges in ophthalmic formulations
Read about the requirements PVA meets for use in ophthalmic preparations and its benefits compared to HPMC and CMC
- Whitepaper: A Closer Look at Polyvinyl Alcohol
Find information on how to overcome challenges in ophthalmic formulations through polymer selection
- Webinar: The Role of Polyvinyl Alcohol (PVA) in Ophthalmic Formulations
Learn about the unique properties of Poly-vinyl alcohol (PVA) relevant for ophthalmics
- Pharma Manufacturing podcast: How to overcome challenges in ophthalmic formulations
About unique challenges and regulatory requirements of ophthalmic drug formulation, and methods to overcome them
- Pharmaceutical Application Guide
Explore products and services for each step of your pharmaceutical manufacturing process
- Formulation Product Finder App
Find the right product for your application with our Formulation Product Finder App
- Risk Mitigation Tool
Get guidance through the challenges and quality requirements of your bio-manufacturing process
Reach out to discuss your ophthalmic drug formulation needs
For additional information or to be contacted by a sales representative, please complete and submit the form below.
To continue reading please sign in or create an account.
Don't Have An Account?