SAE0217
Enhanced Microbial Transglutaminase

Glycosylation tolerant, for site-specific antibody bioconjugation
Synonym(s):
Protein-Glutamine-γ-Glutamyltransferase, Protein-glutamine:amine γ-glutamyltransferase, eMTG
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352204
Recommended Products
recombinant
expressed in E. coli
Quality Level
specific activity
≥30 units/mg protein
shipped in
dry ice
storage temp.
-10 to -25°C
General description
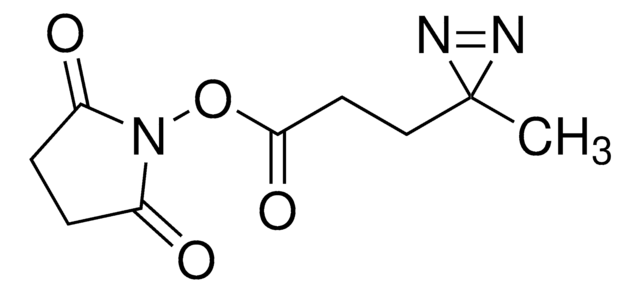
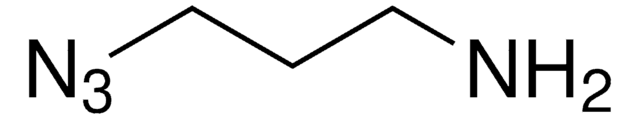
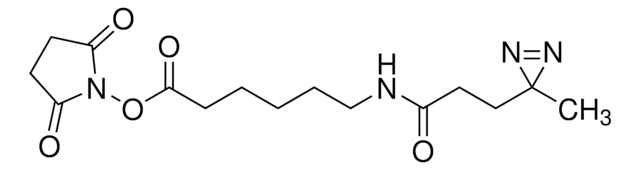
eMTG (Enhanced Microbial Transglutaminase) is an enzyme that catalyzes the formation of new isopeptide bonds between the primary amine of a drug linker and glutamine residues at position Q295 of IgG-type antibodies to produce site-specifically conjugated antibody-drug conjugates (ADCs).
Application
Site-specific conjugation is increasingly used in drug research, development, and manufacturing to produce ADCs and bioconjugates with defined drug-to-antibody ratios (DAR) since the resulting homogeneity improves safety and efficacy. Transglutaminase (TG)-mediated bioconjugation is a promising means of conjugating drug linkers specifically to the Q295 position of antibodies. However, its use traditionally requires antibody or glycan engineering since the adjacent glycosylation at N297 interferes with the TG reaction. If you’re interested in trying eMTG to make therapeutic ADCs but do not currently have the conjugation setup/expertise, please consider ADC Express™ Service for pre-clinical lead candidate selection.
Features and Benefits
• Increased efficiency - with one-step bioconjugation for ADCs
• Glycosylation-tolerant - no need to remove glycosylation allowing for a preserved glycan on the antibody conjugate product
• Built for purpose - eMTG has been designed for producing ADCs and bioconjugates
• For higher-grade quality suitable for cGMP manufacturing of ADCs, please reach out using this form https://www.sigmaaldrich.com/US/en/services/contract-manufacturing/adc-bioconjugation/adc-bioconjugation-request-information with eMTG selected.
• Glycosylation-tolerant - no need to remove glycosylation allowing for a preserved glycan on the antibody conjugate product
• Built for purpose - eMTG has been designed for producing ADCs and bioconjugates
• For higher-grade quality suitable for cGMP manufacturing of ADCs, please reach out using this form https://www.sigmaaldrich.com/US/en/services/contract-manufacturing/adc-bioconjugation/adc-bioconjugation-request-information with eMTG selected.
Unit Definition
One unit will catalyze the formation of 1.0 micromole of hydroxamate per minute from N Z GLN-GLY and hydroxylamine at pH 6.0 at 37°C.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service