P2949
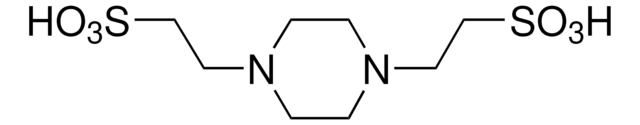
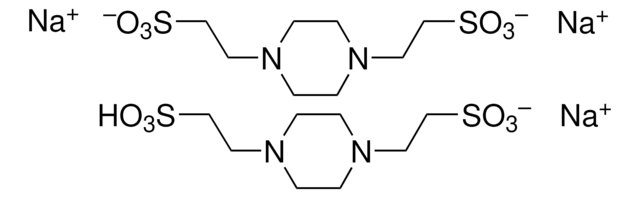
PIPES sodium salt
≥99% (titration)
Synonym(s):
Na-PIPES, Piperazine-1,4-diethanesulfonate sodium salt, 1,4-Piperazinediethanesulfonic acid sodium salt, Piperazine-N,N′-bis(2-ethanesulfonic acid) sodium salt
About This Item
Recommended Products
Quality Level
Assay
≥99% (titration)
form
crystalline powder
pH
4.5-4.7
useful pH range
6.1-7.5
pKa (25 °C)
6.8
solubility
0.1 M NaOH: 250 mg/mL, clear, colorless
application(s)
diagnostic assay manufacturing
storage temp.
room temp
SMILES string
[Na+].OS(=O)(=O)CCN1CCN(CC1)CCS([O-])(=O)=O
InChI
1S/C8H18N2O6S2.Na/c11-17(12,13)7-5-9-1-2-10(4-3-9)6-8-18(14,15)16;/h1-8H2,(H,11,12,13)(H,14,15,16);/q;+1/p-1
InChI key
OGGAIRCLBMGXCZ-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to prepare BRB80 buffer for imaging dynamic microtubules and single kinesin molecules through fluorescence microscopy
- as a component of PEM fixation solution
- to reconstitute red blood cells (RBCs) to study the effect of pH on 5-coordinate alpha-nitrosyl-hemoglobin (HbNO) stability
Features and Benefits
- Non-toxic
- Easy to use
- Thermally stable
- Easy to handle and store
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service