M6517
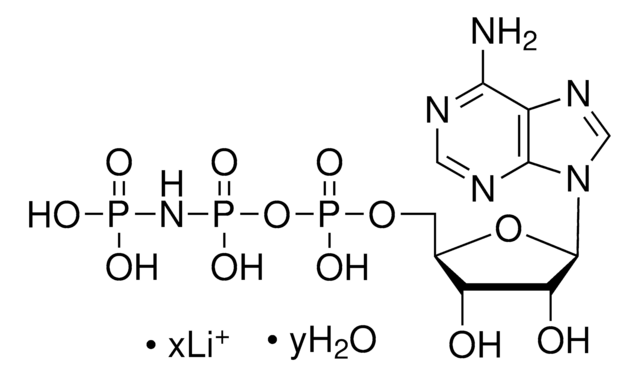
α,β-Methyleneadenosine 5′-triphosphate lithium salt
≥93% (HPLC), solid
Synonym(s):
AMP-CPP
About This Item
Recommended Products
Quality Level
Assay
≥93% (HPLC)
form
solid
color
white
solubility
H2O: 100 mg/mL
storage temp.
−20°C
SMILES string
[Li+].Nc1ncnc2n(cnc12)[C@@H]3O[C@H](COP(O)(=O)CP(O)(=O)OP(O)([O-])=O)[C@@H](O)[C@H]3O
InChI
1S/C11H18N5O12P3.Li/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(27-11)1-26-29(19,20)4-30(21,22)28-31(23,24)25;/h2-3,5,7-8,11,17-18H,1,4H2,(H,19,20)(H,21,22)(H2,12,13,14)(H2,23,24,25);/q;+1/p-1/t5-,7-,8-,11-;/m1./s1
InChI key
NVHVREPTGDOYIC-YCSZXMBFSA-M
Gene Information
human ... P2RX1(5023)
Related Categories
Application
- as P2-purinoreceptor agonist (P2X), to analyse its effects on membrane conductance in the astrocytes of caudal nucleus
- as a P2X receptor agonist, to increase the calcium current in the neurons in satellite cells
- to determine the functional sensitivities of purinergic agonists on P2Y receptors in rat and to determine its effect on developing outer sulcus cells
Biochem/physiol Actions
Features and Benefits
Physical form
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service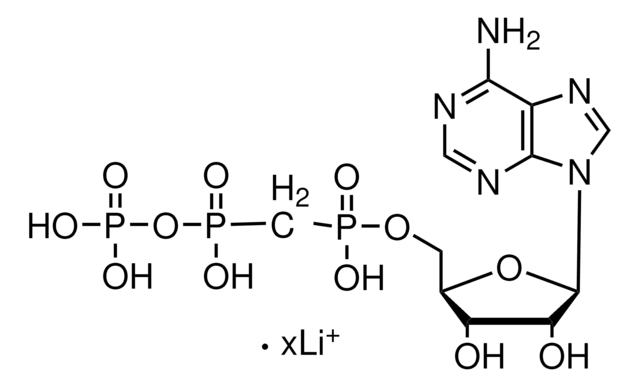


![Adenosine 5′-[γ-thio]triphosphate tetralithium salt ≥75% (HPLC), powder](/deepweb/assets/sigmaaldrich/product/structures/319/398/e29221c2-3649-455b-bd33-583bb017ec7d/640/e29221c2-3649-455b-bd33-583bb017ec7d.png)
![Adenosine 5′-[β-thio]diphosphate trilithium salt ≥80% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/666/651/b097613c-0d79-4acf-9a09-a0a42234d7bd/640/b097613c-0d79-4acf-9a09-a0a42234d7bd.png)