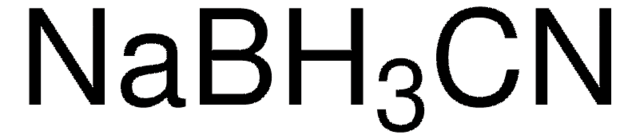C4187
Cyanoborohydride Coupling Buffer
Synonym(s):
Coupling buffer
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12161700
NACRES:
NA.56
Recommended Products
form
liquid
reaction suitability
reagent type: reductant
technique(s)
affinity chromatography: suitable
application(s)
life science and biopharma
storage temp.
2-8°C
Application
A ready-to-use reagent used to couple amine ligands to aldehyde functional groups. The coupling buffer reaction is a reductive amination of the intermediate Schiff′s base to a stable C−N bond.
Cyanoborohydride Coupling Buffer has been used:
- in coupling reactions between amines and glutaraldehyde
- to reduce hydrazone bond to a stable hydrazide bond
- as a component in oligonucleotide reaction mixture for coverslips functionalization
Cyanoborohydride Coupling Buffer is used in affinity chromatography, protein chromatography, activated/functionalized matrices and synthetic reagents. Cyanoborohydride has been used to inform a safe and effective gene-transfer system targeting hepatocytes as well as to develop a method for targeted delivery of anticancer therapeutics to cancer cells in hypoxic areas.
Biochem/physiol Actions
Cyanoborohydride Coupling Buffer is a reagent suitable for reductive amination processes, that contributes to transformation of simple alcohols into more complex amines. It is used in the conversion of Schiff base, by reducing it, to form a secondary amine without affecting aldehyde groups on the support.
Components
0.02 M sodium phosphate, pH 7.5, containing 0.2 M sodium chloride and 3.0 g/L sodium cyanoborohydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Use of polymer supported reagents for clean multi-step organic synthesis: preparation of amines and amine derivatives from alcohols for use in compound library generation
Ley S, et al.
Journal of the Chemical Society. Perkin Transactions 1, 15(27), 2239-2242 (1998)
Christopher A Holden et al.
International journal of nanomedicine, 5, 25-36 (2010-02-18)
Tumors frequently contain hypoxic regions that result from a shortage of oxygen due to poorly organized tumor vasculature. Cancer cells in these areas are resistant to radiation- and chemotherapy, limiting the treatment efficacy. Macrophages have inherent hypoxia-targeting ability and hold
Direct electrical detection of antigen?antibody binding on diamond and silicon substrates using electrical impedance spectroscopy.
Yang W, et al.
Analyst, 132(4), 296-306 (2007)
Binding between the integrin ?X?2 (CD11c/CD18) and heparin.
Vorup-Jensen T, et al.
Test, 282(42), 30869-30877 (2007)
Polymer-supported triacetoxyborohydride: a novel reagent of choice for reductive amination
Bhattacharyya S, et al.
Tetrahedron Letters, 44(27), 4957-4960 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service