C2196
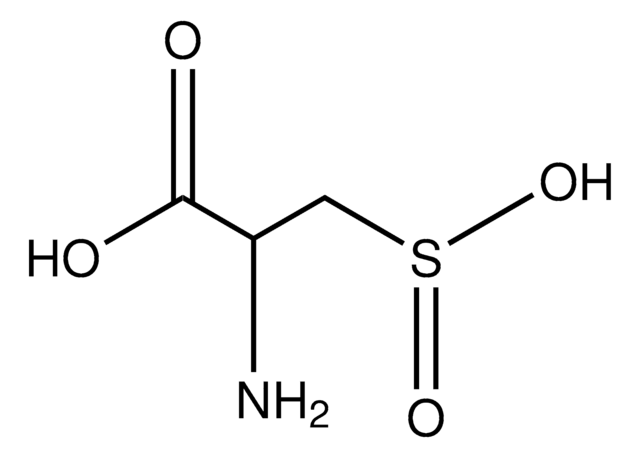
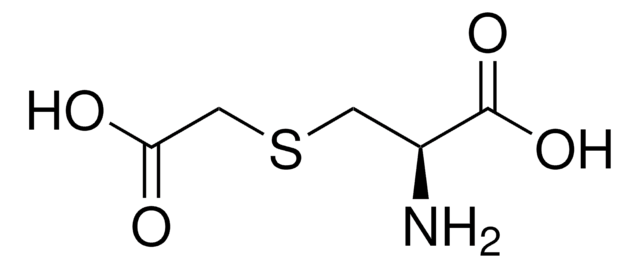
L-Cysteine S-sulfate
≥98% (TLC), suitable for ligand binding assays
Synonym(s):
S-Sulfo-L-cysteine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C3H7NO5S2
CAS Number:
Molecular Weight:
201.22
Beilstein:
1726832
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
L-Cysteine S-sulfate, ≥98% (TLC)
Quality Level
Assay
≥98% (TLC)
form
powder
technique(s)
ligand binding assay: suitable
color
white
storage temp.
−20°C
SMILES string
N[C@@H](CSS(O)(=O)=O)C(O)=O
InChI
1S/C3H7NO5S2/c4-2(3(5)6)1-10-11(7,8)9/h2H,1,4H2,(H,5,6)(H,7,8,9)/t2-/m0/s1
InChI key
NOKPBJYHPHHWAN-REOHCLBHSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
L-Cysteine S-sulfate (LCSS) is used as an NMDA glutamatergic receptor agonist. It serves as a substrate for cystine lyase(s).
Cysteine is one of the functional amino acids that are associated with growth, reproduction, maintenance and immunity. Cysteine is a source of disulfide linkage in protein and is associated with sulfur transport. At physiological pH, cysteine undergoes rapid oxidation to form cystine. Reduced availability of cysteine or cystine, influences leukocyte metabolism. L-Cysteine serves as a precursor for the rate limiting step in glutathione synthesis that occurs in neurons. It donates inorganic sulfate for detoxification reactions. Therefore, L-cysteine might be associated with neuroprotection. It is found to obstruct the entry of heavy metal ions across the blood-brain barrier into the brain. Increased levels of L-cysteine might lead to neurotoxicity.
Cysteine is one of the functional amino acids that are associated with growth, reproduction, maintenance and immunity. Cysteine is a source of disulfide linkage in protein and is associated with sulfur transport. At physiological pH, cysteine undergoes rapid oxidation to form cystine. Reduced availability of cysteine or cystine, influences leukocyte metabolism. L-Cysteine serves as a precursor for the rate limiting step in glutathione synthesis that occurs in neurons. It donates inorganic sulfate for detoxification reactions. Therefore, L-cysteine might be associated with neuroprotection. It is found to obstruct the entry of heavy metal ions across the blood-brain barrier into the brain. Increased levels of L-cysteine might lead to neurotoxicity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
W Xia et al.
Journal of pharmaceutical and biomedical analysis, 19(1-2), 261-268 (2000-03-04)
Neonatal Sprague Dawley rat brain tissue was extracted with methanol, acetonitrile, acetic acid and trifluoroacetic acids (TFA). Among the extractants tested, 0.1 M TFA gave the highest recovery, 73.4 +/- 5.2% (slope of regression of 'added' vs. 'found' and standard
F Orrego et al.
Neuroscience, 56(3), 539-555 (1993-10-01)
The chemical nature of the central transmitter responsible for fast excitatory events and other related phenomena is analysed against the historical background that has progressively clarified the structure and function of central synapses. One of the problems posed by research
Emma J Footitt et al.
Journal of inherited metabolic disease, 34(2), 529-538 (2011-02-10)
Analysis of pyridoxal 5'-phosphate (PLP) concentration in 256 cerebrospinal fluid (CSF) samples from patients with neurological symptoms showed that the variance is greater than indicated by previous studies. The age-related lower reference limit has been revised to detect inborn errors
Desensitization of NMDA receptor channels is modulated by glutamate agonists.
Nahum-Levy R
Biophysical Journal, 80(5), 2152-2166 (2001)
Takeshi Nakatani et al.
Microbial cell factories, 11, 62-62 (2012-05-23)
Escherichia coli has two L-cysteine biosynthetic pathways; one is synthesized from O-acetyl L-serine (OAS) and sulfate by L-cysteine synthase (CysK), and another is produced via S-sulfocysteine (SSC) from OAS and thiosulfate by SSC synthase (CysM). SSC is converted into L-cysteine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service