A3626
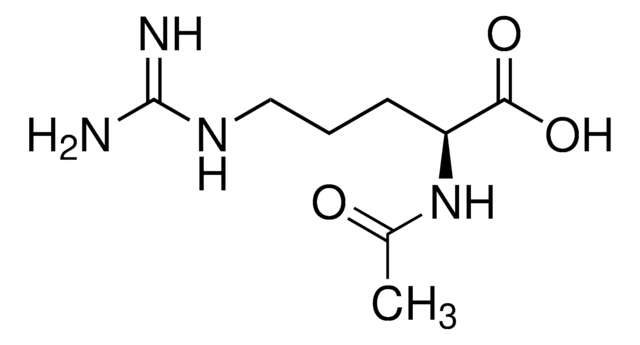
Nα-Acetyl-L-ornithine
≥98% (TLC), suitable for ligand binding assays
Synonym(s):
N2-acetyl-L-lysine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H14N2O3
CAS Number:
Molecular Weight:
174.20
MDL number:
UNSPSC Code:
12352202
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
Nα-Acetyl-L-ornithine,
Assay
≥98% (TLC)
Quality Level
form
powder
technique(s)
ligand binding assay: suitable
color
colorless to white
storage temp.
−20°C
SMILES string
CC(=O)N[C@@H](CCCN)C(O)=O
InChI
1S/C7H14N2O3/c1-5(10)9-6(7(11)12)3-2-4-8/h6H,2-4,8H2,1H3,(H,9,10)(H,11,12)/t6-/m0/s1
InChI key
JRLGPAXAGHMNOL-LURJTMIESA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
Nα-Acetyl-L-ornithine (AORN) is a substrate for the identification, differentiation and characterization of N(α)-acetyl-L-ornithine deacetylase(s) and of N-Acetyl-l-ornithine transcarbamylase(s) (AOTCase) found in plants, some eubacteria and some human pathogens.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Daniel Charlier et al.
Amino acids, 51(8), 1103-1127 (2019-07-04)
Already very early, the study of microbial arginine biosynthesis and its regulation contributed significantly to the development of new ideas and concepts. Hence, the term "repression" was proposed by Vogel (The chemical basis of heredity, The John Hopkins Press, Baltimore
Séverine Lemarié et al.
Plant & cell physiology, 56(11), 2158-2168 (2015-09-13)
The role of salicylic acid (SA) and jasmonic acid (JA) signaling in resistance to root pathogens has been poorly documented. We assessed the contribution of SA and JA to basal and partial resistance of Arabidopsis to the biotrophic clubroot agent
Dashuang Shi et al.
Proteins, 64(2), 532-542 (2006-06-03)
N-acetyl-L-ornithine transcarbamoylase (AOTCase) is a new member of the transcarbamoylase superfamily that is essential for arginine biosynthesis in several eubacteria. We report here crystal structures of the binary complexes of AOTCase with its substrates, carbamoyl phosphate (CP) or N-acetyl-L-ornithine (AORN)
Dashuang Shi et al.
The Journal of biological chemistry, 280(15), 14366-14369 (2005-02-26)
We have identified in Xanthomonas campestris a novel N-acetylornithine transcarbamylase that replaces ornithine transcarbamylase in the canonic arginine biosynthetic pathway of several Eubacteria. The crystal structures of the protein in the presence and absence of the reaction product, N-acetylcitrulline, were
Yongdong Li et al.
Biochemistry, 49(32), 6887-6895 (2010-08-11)
N-Acetyl-l-ornithine transcarbamylase (AOTCase), rather than ornithine transcarbamylase (OTCase), is the essential carbamylase enzyme in the arginine biosynthesis of several plant and human pathogens. The specificity of this unique enzyme provides a potential target for controlling the spread of these pathogens.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service