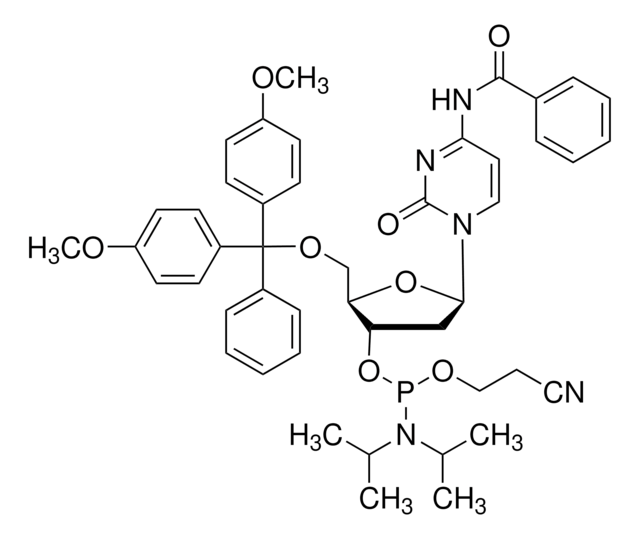
A112000
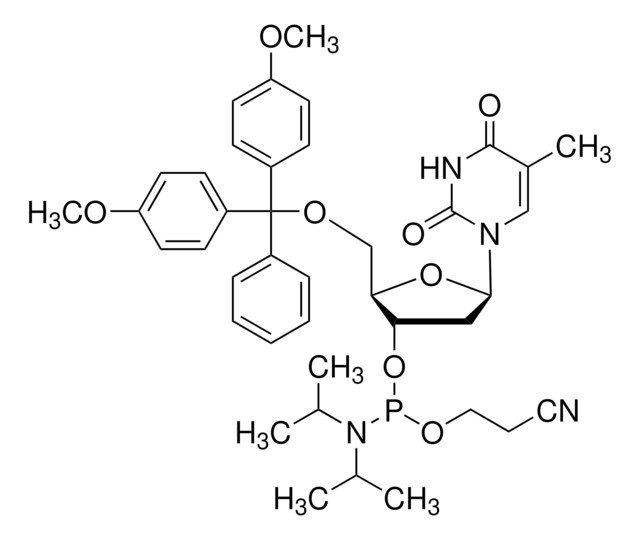
DMT-dA(tac) Phosphoramidite
Synonym(s):
DMT-dA(tac) amidite
About This Item
Recommended Products
type
for DNA synthesis
Quality Level
product line
Proligo Reagents
Assay
≥98% (31P-NMR)
≥98.0% (reversed phase HPLC)
form
powder
technique(s)
oligo synthesis: suitable
λ
conforms (UV/VIS Identity)
nucleoside profile
base: deoxyadenosine
base protecting group: TAC
2' protecting group: none
5' protecting group: DMT
deprotection: fast
storage temp.
-10 to -25°C
SMILES string
COc1ccc(cc1)C(OC[C@H]2O[C@H](C[C@@H]2OP(OCCC#N)N(C(C)C)C(C)C)n3cnc4c(NC(=O)COc5ccccc5)ncnc34)(c6ccccc6)c7ccc(OC)cc7
InChI
1S/C48H54N7O8P/c1-33(2)55(34(3)4)64(61-27-13-26-49)63-41-28-44(54-32-52-45-46(50-31-51-47(45)54)53-43(56)30-59-40-16-11-8-12-17-40)62-42(41)29-60-48(35-14-9-7-10-15-35,36-18-22-38(57-5)23-19-36)37-20-24-39(58-6)25-21-37/h7-12,14-25,31-34,41-42,44H,13,27-30H2,1-6H3,(H,50,51,53,56)/t41-,42+,44+,64?/m0/s1
InChI key
INUFZOANRLMUCW-SDNJGBRXSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Key Features of TAC Chemistry are -
- Deprotection of the TAC group is ultra-fast: complete deprotection in concentrated ammonia occurs within 15 minutes at 55 °C or two hours at room temperature
- Compatible with the AMA deprotection reagent (a mixture of ≥25% ammonia in water with 40% aqueous methylamine I/I, v/v)
- Highly soluble in acetonitrile. No need to add co-solvents such as dimethylformamide or methylene chloride
- Suitable for the synthesis of oligomers with base-labile units e.g., dyes and modifiers, because of less exposure to ammonia and the possibility of room temperature deprotection
- No change is required in the reagents commonly used for DNA synthesis, except that Proligo′s Fast Deprotection Cap A solution is used instead of Cap A solution •
- The application of dA(tac) minimizes depurination and improves the quality of oligonucleotides
Features and Benefits
- The deprotection of oligonucleotide synthesis products with the AMAreagent is ultra-fast: complete deprotection requires 10 minutes at 65 °C
- Side reactions at C-monomers through transamination are eliminated
- Not compatible with some base-labile modified nucleosides
- dC(tac)-phosphoramidite can directly substitute for dC(bz)-phosphoramidite
- No change is required in the reagents commonly used for DNA synthesis.Acetonitrile is used to dissolve phosphoramidite. The standard aceticanhydride capping reagent can be employed.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service