A0876
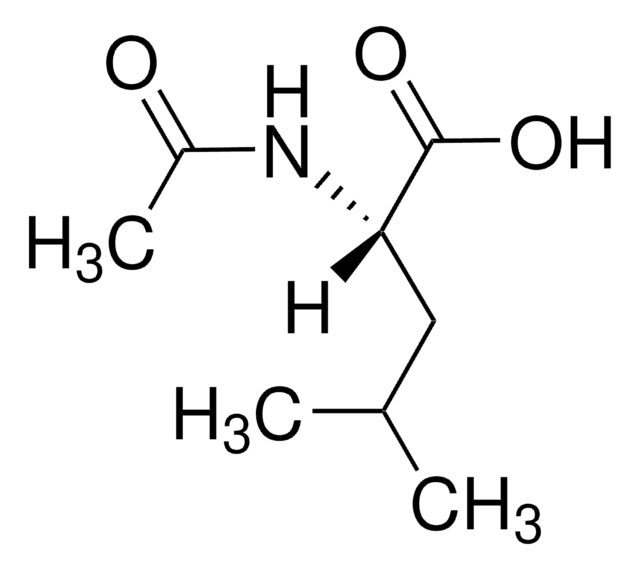
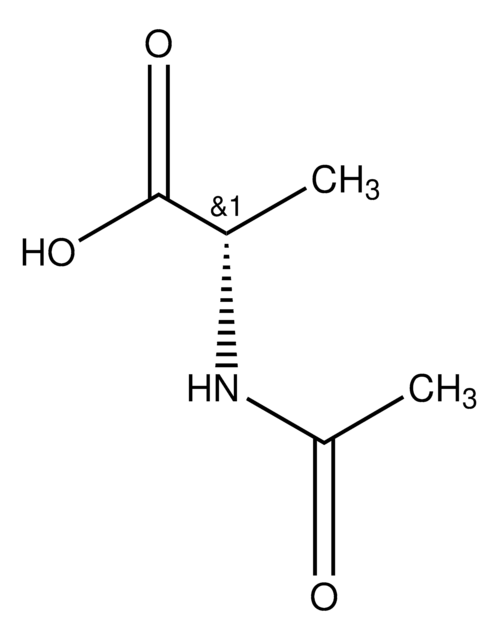
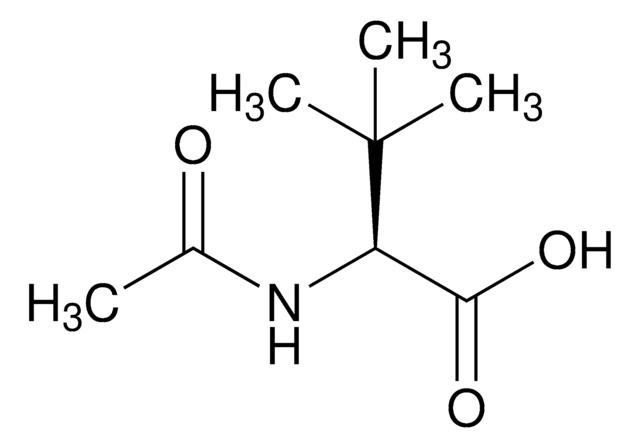
N-Acetyl-D-leucine
≥99% (TLC), suitable for ligand binding assays and cell cutlure
Synonym(s):
N-acetyl-D-Leucine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H15NO3
CAS Number:
Molecular Weight:
173.21
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
N-Acetyl-D-leucine,
Assay
≥99% (TLC)
Quality Level
form
powder
technique(s)
cell culture | mammalian: suitable
ligand binding assay: suitable
color
white
storage temp.
−20°C
SMILES string
CC(C)C[C@@H](NC(C)=O)C(O)=O
InChI
1S/C8H15NO3/c1-5(2)4-7(8(11)12)9-6(3)10/h5,7H,4H2,1-3H3,(H,9,10)(H,11,12)/t7-/m1/s1
InChI key
WXNXCEHXYPACJF-SSDOTTSWSA-N
Application
N-Acetyl-D-leucine may be used with other D-aminoacylated amino acids as a substrate for the identification, differentiation and characterization of D-aminoacylase(s)/amidohydrolase(s).
Biochem/physiol Actions
N-Acetyl-D-leucine is a substrate for D-aminoacylase from Alcaligenes xylosoxydans subsp. xylosoxydans A-6. N-Acetyl-D-leucine is used to help differentiate members of the amidohydrolase enzyme superfamily. It is a preferred substrate of Gox1177 from Gluconobacter oxidans.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Y B Yang et al.
Bioscience, biotechnology, and biochemistry, 56(9), 1392-1395 (1992-09-01)
The D-aminoacylase produced by Alcaligenes denitrificans DA181 was a new type of aminoacylase which had both high stereospecificity and specific activity. The molecular weight and isoelectric point of this enzyme were 58,000 and 4.4, respectively. The apparent Km and kcat
M Moriguchi et al.
Bioscience, biotechnology, and biochemistry, 57(7), 1149-1152 (1993-07-01)
The best inducers for D-aminoacylase from Alcaligenes xylosoxydans subsp. xylosoxydans A-6 (Alcaligenes A-6) were a poor substrate, N-acetyl-gamma-methyl-D-leucine, and an inhibitor, N-acetyl-D-alloisoleucine. The enzyme has been homogeneously purified. The molecular weight of the native enzyme was estimated to be 58,000
Jennifer A Cummings et al.
Biochemistry, 48(27), 6469-6481 (2009-06-13)
The catalytic activities of three members of the amidohydrolase superfamily were discovered using amino acid substrate libraries. Bb3285 from Bordetella bronchiseptica, Gox1177 from Gluconobacter oxidans, and Sco4986 from Streptomyces coelicolor are currently annotated as d-aminoacylases or N-acetyl-d-glutamate deacetylases. These three
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service