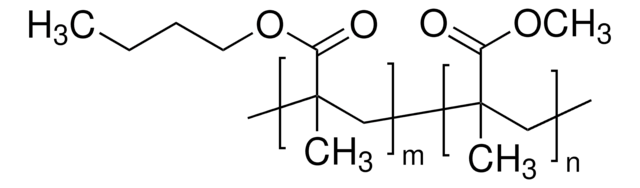
66939
Phalloidin–Atto 425
suitable for fluorescence, ≥90% (HPLC)
Synonym(s):
Atto 425–Phalloidin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352116
NACRES:
NA.32
Recommended Products
Assay
≥90% (HPLC)
manufacturer/tradename
ATTO-TEC GmbH
λ
in methanol
UV absorption
λ: 429-435 nm Amax
suitability
suitable for fluorescence
storage temp.
−20°C
General description
Atto 425 Phalloidin is a novel fluorescent label with a coumarin structure. The dye is intended for application in the area of life science, e.g. labeling of DNA, RNA or proteins. Characteristic features of the label are strong absorption, high fluorescence quantum yield, large Stokes-shift, good photostability, and low molecular weight.Phalloidin is a fungal toxin isolated from the poisonous mushroom Amanita phalloides. Its toxicity is attributed to the ability to bind F actin in liver and muscle cells. As a result of binding phalloidin, actin filaments become strongly stabilized. Phalloidin has been found to bind only to polymeric and oligomeric forms of actin, and not to monomeric actin. The dissociation constant of the actin-phalloidin complex has been determined to be on the order of 3 x 10–8. Phalloidin differs from amanitin in rapidity of action; at high dose levels, death of mice or rats occurs within 1 or 2 hours.
Application
Fluorescent conjugates of phalloidin, rhodamine-phalloidin staining reagents, such as Phalloidin–Atto 425 are used to label actin filaments for histological applications. Some structural features of phalloidin are required for the binding to actin. However, the side chain of amino acid 7 (g-dihydroxyleucine) is accessible for chemical modifications without appreciable loss of affinity for actin.
Legal Information
This product is for Research use only. In case of intended commercialization, please contact the IP-holder (ATTO-TEC GmbH, Germany) for licensing.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Louise R Page
Evolution & development, 4(3), 212-222 (2002-06-11)
Results of this study on two species of vetigastropods contradict the long-standing hypothesis, originally proposed by Garstang (1929), that the larval retractor muscles power the morphogenetic movement of ontogenetic torsion in all basal gastropods. In the trochid Calliostoma ligatum and
Rosangela Invernizzi et al.
Oncology, 75(3-4), 237-244 (2008-10-16)
Pegfilgrastim is a covalent conjugate of filgrastim and polyethylene glycol that has proved to be effective in supporting myelopoiesis during chemotherapy. Since very limited information is available on the biological effects of pegfilgrastim on neutrophils exposed to chemotherapy, we analyzed
Takahiro Shimizu et al.
Cell calcium, 45(3), 226-232 (2008-12-03)
We demonstrate here that the transient receptor potential melastatin subfamily channel, TRPM4, controls migration of bone marrow-derived mast cells (BMMCs), triggered by dinitrophenylated human serum albumin (DNP-HSA) or stem cell factor (SCF). Wild-type BMMCs migrate after stimulation with DNP-HSA or
Stimulated emission depletion-based raster image correlation spectroscopy reveals biomolecular dynamics in live cells.
Hedde P.N.; et al.
Nature Communications, 4, 2093-2093 (2013)
SNARE Function Is Not Involved in Early Endosome Docking.
Geumann, U.; et al.
Molecular Biology of the Cell, 19(12), 5327-5337 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service