92688
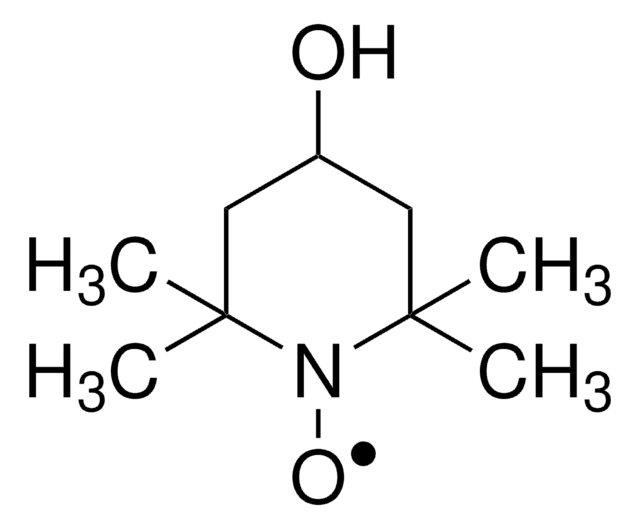
5,5-Dimethyl-1-pyrroline N-oxide
for ESR-spectroscopy
Synonym(s):
DMPO
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11NO
CAS Number:
Molecular Weight:
113.16
Beilstein:
107603
EC Number:
MDL number:
UNSPSC Code:
12000000
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
grade
for ESR-spectroscopy
Quality Level
Assay
≥98.0% (GC)
form
crystals
refractive index
n20/D 1.496 (lit.)
bp
75 °C/0.4 mmHg (lit.)
mp
25-29 °C (lit.)
density
1.015 g/mL at 25 °C (lit.)
storage temp.
−20°C
SMILES string
CC1(C)CCC=[N+]1[O-]
InChI
1S/C6H11NO/c1-6(2)4-3-5-7(6)8/h5H,3-4H2,1-2H3
InChI key
VCUVETGKTILCLC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5,5-Dimethyl-1-pyrroline N-oxide is a reagent generally used either as a free-radical spin-trapping agent, or electrophilic component during the synthesis of pyrrolidine derivatives. It may also be considered as 1,3-dipole in cycloaddition processes.
Neuroprotective agent; nitric oxide spin trap. Used to study radicals formed by enzymatic acetaldehyde oxidation. Incubation of lymphocytes with DMPO decreased DNA damage by NiCl2.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
203.0 °F - closed cup
Flash Point(C)
95 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Karim Michail et al.
Analytical chemistry, 84(15), 6739-6746 (2012-06-26)
Free radicals are conventionally detected by electron paramagnetic resonance (EPR) spectroscopy after being trapped as spin adducts. Albeit this technique has demonstrated utmost efficacy in studying free radicals, its application to biological settings is intrinsically hampered by the inevitable bioreduction
Murugesan Velayutham et al.
Free radical biology & medicine, 51(1), 160-170 (2011-05-07)
In cells, mitochondria, endoplasmic reticulum, and peroxisomes are the major sources of reactive oxygen species (ROS) under physiological and pathophysiological conditions. Cytochrome c (cyt c) is known to participate in mitochondrial electron transport and has antioxidant and peroxidase activities. Under
Pedro L Zamora et al.
The journal of physical chemistry. A, 116(26), 7210-7218 (2012-06-07)
Radical forms of sulfur dioxide (SO(2)), sulfite (SO(3)(2-)), sulfate (SO(4)(2-)), and their conjugate acids are known to be generated in vivo through various chemical and biochemical pathways. Oxides of sulfur are environmentally pervasive compounds and are associated with a number
Emiko Sato et al.
Journal of biochemistry, 150(2), 173-181 (2011-05-17)
The nicotinamide adenine dinucleotide (NADH)/nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and the xanthine oxidase (XOD) systems generate reactive oxygen species (ROS). In the present study, to characterize the difference between the two systems, the kinetics of ROS generated by both
Suchandra Bhattacharjee et al.
Nucleic acids research, 40(12), 5477-5486 (2012-03-06)
Oxidative stress-related damage to the DNA macromolecule produces lesions that are implicated in various diseases. To understand damage to DNA, it is important to study the free radical reactions causing the damage. Measurement of DNA damage has been a matter
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service