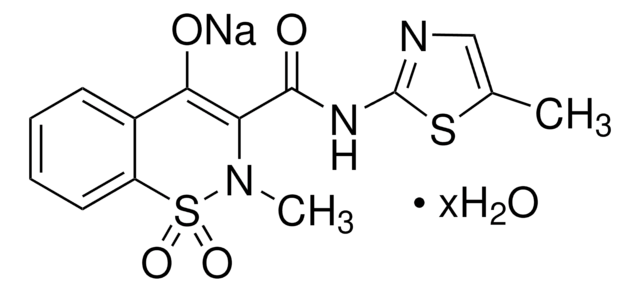
33586
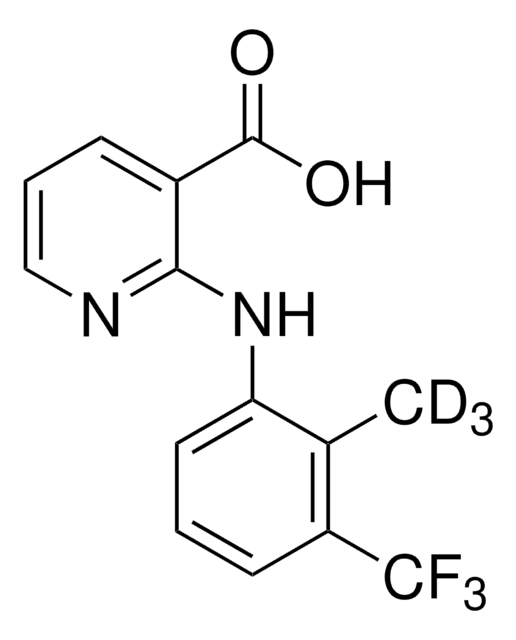
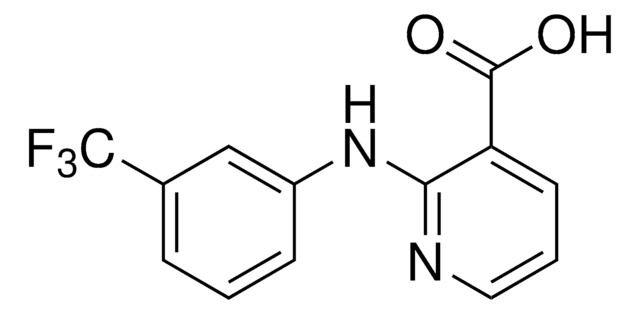
Flunixin
VETRANAL®, analytical standard
Synonym(s):
2-[2-Methyl-3-(trifluoromethyl)phenylamino]nicotinic acid
About This Item
Recommended Products
grade
analytical standard
Quality Level
product line
VETRANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
forensics and toxicology
pharmaceutical (small molecule)
format
neat
SMILES string
Cc1c(Nc2ncccc2C(O)=O)cccc1C(F)(F)F
InChI
1S/C14H11F3N2O2/c1-8-10(14(15,16)17)5-2-6-11(8)19-12-9(13(20)21)4-3-7-18-12/h2-7H,1H3,(H,18,19)(H,20,21)
InChI key
NOOCSNJCXJYGPE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Bovine muscle samples by hydrophilic interaction liquid chromatography-electrospray-tandem mass spectrometry (HILIC-ESI-MS/MS) equipped with selected reaction monitoring (SRM) detection.
- Animal tissues by dispersive-solid phase extraction (d-SPE) and enhanced matrix removal for lipids (EMR-L), followed by analysis using ultra-high performance liquid chromatography-triple quadrupole or quadrupole-time-of-flight (UHPLC-QqQ or UHPLC-Q/TOF) methods in conjunction with ESI-MS/MS operating on multiple reaction monitoring (MRM) mode of detection as well as ESI-LC-MS/MS with SRM detection mode.
- Porcine muscle samples by ESI-LC-MS/MS with MRM detection mode.
Recommended products
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service