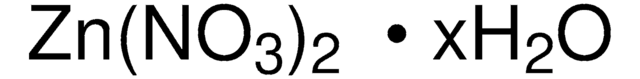28-5730
Sulfanilamide
JIS special grade, ≥99.7%
Synonym(s):
p-Aminobenzenesulfonamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC6H4SO2NH2
CAS Number:
Molecular Weight:
172.20
Beilstein:
511852
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
grade:
JIS special grade
form:
solid
Recommended Products
grade
JIS special grade
Assay
≥99.7%
form
solid
availability
available only in Japan
mp
164-166 °C (lit.)
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
Mode of action
DNA synthesis | interferes
enzyme | inhibits
SMILES string
Nc1ccc(cc1)S(N)(=O)=O
InChI
1S/C6H8N2O2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H2,8,9,10)
InChI key
FDDDEECHVMSUSB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Chemical structure: sulfonamide
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xian-Long Wang et al.
European journal of medicinal chemistry, 45(10), 4631-4639 (2010-08-17)
A series of novel sulfanilamide-derived 1,2,3-triazole compounds were synthesized in excellent yields via 1,3-dipolar cycloaddition and confirmed by MS, IR and NMR spectra as well as elemental analyses. All the compounds were screened in vitro for their antibacterial and antifungal
Fei Liu et al.
Organic letters, 12(4), 868-871 (2010-01-19)
The synthesis of benzofused sultams and/or fluorinated sulfonamides, starting from N-allylic sulfonamides, was performed in superacid HF/SbF(5), through superelectrophilic activation. A dramatic effect of superacid composition on the selectivity of the reaction was shown and applied to the synthesis of
Ramazan Demirdağ et al.
Bioorganic & medicinal chemistry, 21(6), 1522-1525 (2012-09-15)
Carbonic anhydrase (CA, EC: 4.2.1.1) was purified from sheep kidney by affinity chromatography on a Sepharose 4B-tyrosine-sulfanilamide column. By means of two consecutive procedures, the enzyme (sCA) was purified 227.61-fold with a yield of 60.75%, and a specific activity of
Patricia Texeira Santana et al.
Journal of innate immunity, 7(4), 417-427 (2015-02-14)
Sepsis is associated with high mortality rates in intensive care units worldwide and represents a systemic inflammatory response to infection. P2X7 is an ionotropic purine receptor with known proinflammatory activity. Here, we investigated the role of the P2X7 receptor in
Sevgi Kolayli et al.
Journal of enzyme inhibition and medicinal chemistry, 26(6), 895-900 (2011-03-09)
An α-carbonic anhydrase (CA, EC 4.2.1.1) was purified and characterized kinetically from erythrocytes of the sturgeon Acipenser gueldenstaedti, an endangered species. The sturgeon enzyme (AgCA) showed kinetic parameters for the CO(2) hydration reaction comparable with those of the human erythrocytes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service