T80500
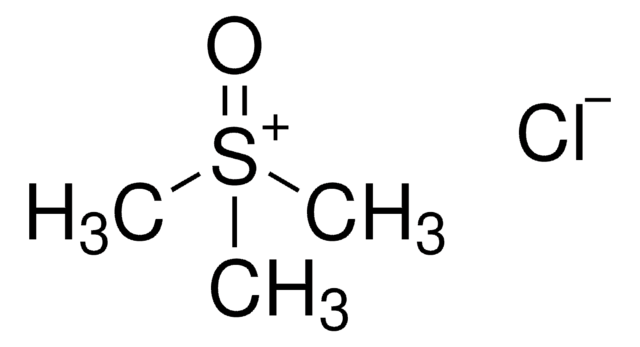
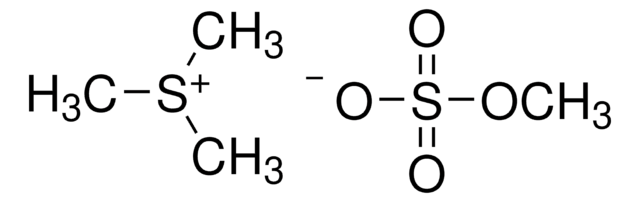
Trimethylsulfoxonium iodide
98%
Synonym(s):
Iodotrimethyloxosulfur, S,S,S-Trimethylsulfoxonium iodide, Trimethyl(oxo)-λ6-sulfanylium iodide, Trimethyloxosulfonium iodide, Trimethyloxosulphonium iodide, Trimethylsulfoxonium iodide (7CI)
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)3S(I)O
CAS Number:
Molecular Weight:
220.07
Beilstein:
3595854
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
208-212 °C (dec.) (lit.)
SMILES string
[I-].C[S+](C)(C)=O
InChI
1S/C3H9OS.HI/c1-5(2,3)4;/h1-3H3;1H/q+1;/p-1
InChI key
BPLKQGGAXWRFOE-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Treatment with strong base yields the ylide which adds to the carbonyl group of ketones and aldehydes to give epoxides. Also adds preferentially to the double bond of α,β-unsaturated esters to give cyclopropyl esters.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Optical phonons, crystal-field transitions, and europium luminescence-excitation processes in Eu2BaCoO5: Experiment and theory.
Taboada et al.
Physical review. B, Condensed matter, 50(13), 9157-9168 (1994-10-01)
W T Ashton et al.
Journal of medicinal chemistry, 35(11), 2103-2112 (1992-05-29)
A series of transition-state analogues having heterocyclythio C-termini has been synthesized and evaluated for inhibition of human renin. Addition of mercaptoheterocycles to a chiral Boc-amino epoxide intermediate led, after several steps, to the target [(2R,3S)-3-(BocPheHis-amino)-4-cyclohexyl-2-hydroxy-1-butyl]thio derivatives. Oxidation of the thioether
Tetrahedron, 49, 5067-5067 (1993)
Tetrahedron, 48, 5089-5089 (1992)
The Journal of Organic Chemistry, 58, 3148-3148 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service