M6204
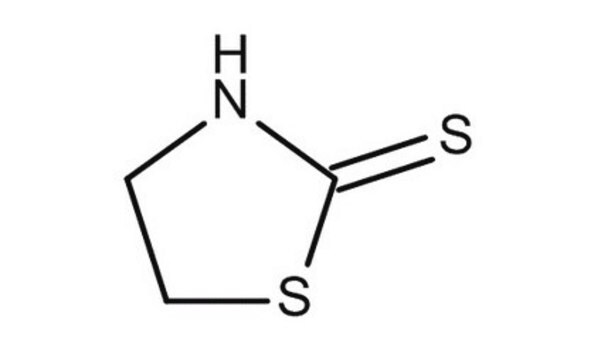
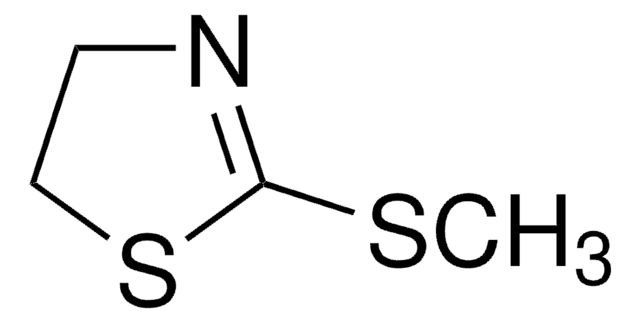
2-Thiazoline-2-thiol
98%
Synonym(s):
2-Mercapto-2-thiazoline
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H5NS2
CAS Number:
Molecular Weight:
119.21
Beilstein:
106332
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
100-105 °C (lit.)
SMILES string
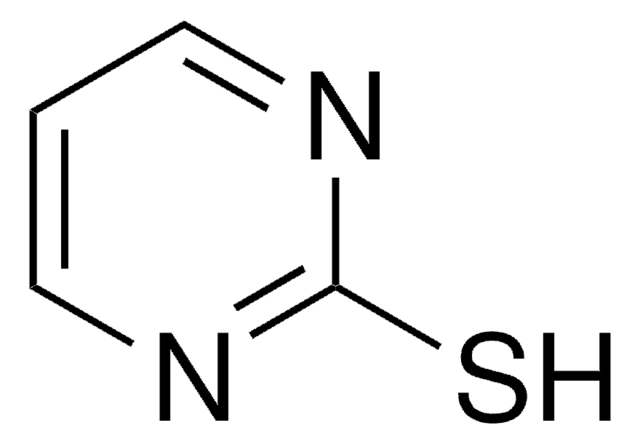
S=C1NCCS1
InChI
1S/C3H5NS2/c5-3-4-1-2-6-3/h1-2H2,(H,4,5)
InChI key
WGJCBBASTRWVJL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Tool for highly selective chiral syntheses of penam- and carbapenam-type β-lactam antibiotics.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chem. Abstr., 108, 37417r-37417r (1988)
Stud. Org. Chem., 28, 57-57 (1987)
Yahia N Mabkhot et al.
Molecules (Basel, Switzerland), 24(9) (2019-05-01)
A series of new thiazoline derivatives were synthesized. Structure analyses were accomplished employing 1H-NMR, 13C-NMR, X-ray and MS techniques. The in vitro antitumor activities were assessed against human hepatocellular carcinoma (HepG-2) and colorectal carcinoma (HCT-116) cell lines. The results revealed
R B Greenwald et al.
Bioconjugate chemistry, 7(6), 638-641 (1996-11-01)
A novel PEG linker that employs a thiazolidine-2-thione group has been synthesized. Kinetic studies done on this compound demonstrate a relatively long half-life compared to those of traditional succinimidyl linkers. This new PEG derivative reacts with proteins under mild conditions
Denis L Guerra et al.
Journal of hazardous materials, 183(1-3), 81-86 (2010-08-03)
The synthetic imogolite sample was used for organofunctionalization process with 2-mercaptothiazoline (MTZ). The compound 2-mercaptothiazoline was anchored onto imogolite surface by heterogeneous route. Due to the increment of basic centers attached to the pendant chains the dye adsorption capability of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service