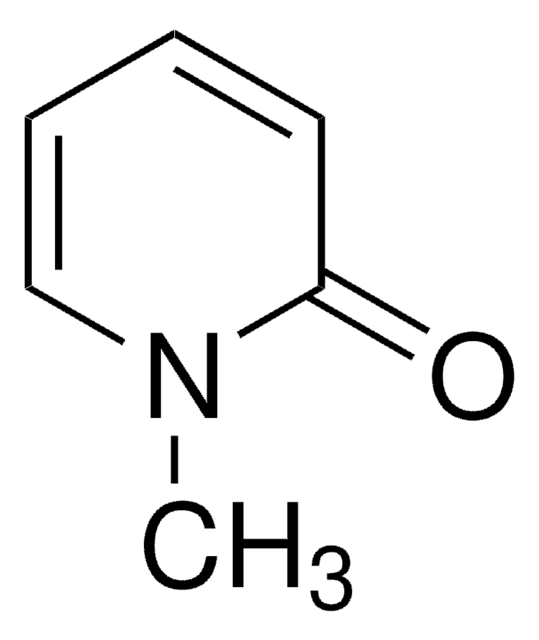
H57009
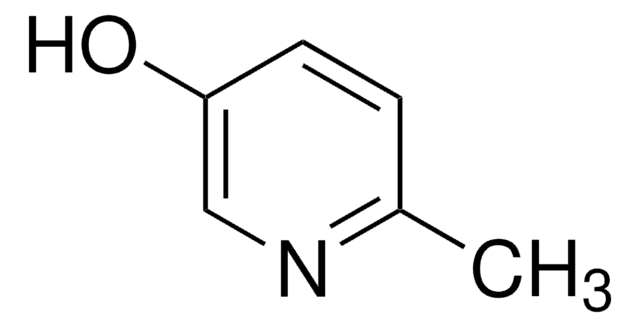
3-Hydroxypyridine
98%
Synonym(s):
3-Pyridinol, 3-Pyridone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5NO
CAS Number:
Molecular Weight:
95.10
Beilstein:
105699
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
125-128 °C (lit.)
SMILES string
Oc1cccnc1
InChI
1S/C5H5NO/c7-5-2-1-3-6-4-5/h1-4,7H
InChI key
GRFNBEZIAWKNCO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L D Lukyanova et al.
Bulletin of experimental biology and medicine, 148(4), 587-591 (2010-04-17)
Succinate-containing derivatives of 3-hydroxypyridine, mexidol and proxypin, serve as succinate donors for the respiratory chain and contribute to activation of the succinate oxidase pathway of oxidation. Under conditions of hypoxia, these changes promote recovery of aerobic energy production, normalization of
Georg T Wondrak et al.
The Journal of biological chemistry, 279(29), 30009-30020 (2004-05-11)
Photocarcinogenesis and photoaging are established consequences of chronic exposure of human skin to solar irradiation. Accumulating evidence supports a causative involvement of UVA irradiation in skin photo-damage. UVA photodamage has been attributed to photosensitization by endogenous skin chromophores leading to
Fazlul Huq et al.
European journal of medicinal chemistry, 39(8), 691-697 (2004-07-28)
Four trans-planaramineplatinum(II) complexes code named YH9, YH10, YH11 and YH12, each of the form trans-PtL(NH(3))Cl(2) where L = 2-hydroxypyridine and 3-hydroxypyridine, imidazole, and imidazo(1,2-alpha)pyridine for YH9, YH10, YH11 and YH12, respectively. All of the compounds have significant anticancer activity against
Zhongfa Liu et al.
Chemical research in toxicology, 16(2), 232-241 (2003-02-18)
2-hydroxyaldehydes have been previously identified as products of lipid peroxidation, and although they represent the simplest reducing sugars, their potential for modification of proteins under physiological conditions has not been investigated. Here, 2-hydroxyaldehydes were found to condense with amines in
E Kindt et al.
Analytical biochemistry, 283(1), 71-76 (2000-08-10)
Preclinical efficacy testing commonly involves studies that require considerable resources and time. One valuable tool in this endeavor is the characterization of relevant biomarkers. A method has been developed for the simultaneous determination of collagen biomarker candidates as an instrument
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service