E12907
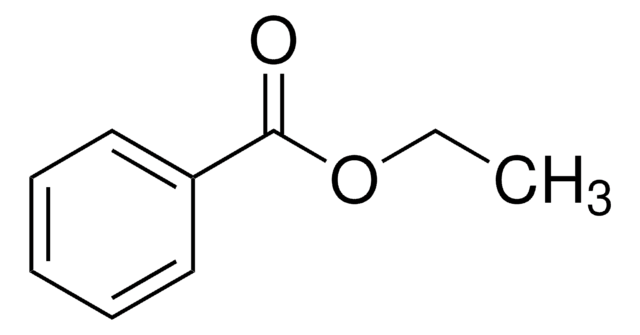
Ethyl benzoate
≥99%
Synonym(s):
Benzoic acid ethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H5COOC2H5
CAS Number:
Molecular Weight:
150.17
Beilstein:
1908172
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
5.17 (vs air)
vapor pressure
1 mmHg ( 44 °C)
Assay
≥99%
form
liquid
autoignition temp.
914 °F
refractive index
n20/D 1.504 (lit.)
bp
212 °C (lit.)
mp
−34 °C (lit.)
density
1.045 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)c1ccccc1
InChI
1S/C9H10O2/c1-2-11-9(10)8-6-4-3-5-7-8/h3-7H,2H2,1H3
InChI key
MTZQAGJQAFMTAQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Ethyl benzoate is a general reagent to construct molecules with phenyl pendants such as phenyl bearing pyrazine-boron fluorescent complex. It can be used to prepare Horner′s phosphonate intermediate in the total synthesis of diospongins A and B. It is also a flavoring agent used in food and fragrance industry.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
190.4 °F - closed cup
Flash Point(C)
88 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A concise total synthesis of diospongins A and B.
Sabitha G, et al.
Helvetica Chimica Acta, 91(12), 2235-2239 (2008)
Densities and viscosities of binary mixtures of isoamyl acetate, ethyl caproate, ethyl benzoate, isoamyl butyrate, ethyl phenylacetate, and ethyl caprylate with ethanol at T=(288.15, 298.15, 308.15, and 318.15) K.
Sheu Y W and Tu C H
Journal of Chemical and Engineering Data, 51(2), 496-503 (2006)
Synthesis and fluorescence properties of novel pyrazine?boron complexes bearing a ?-iminoketone ligand.
Kubota Y, et al.
Organic Letters, 13(24), 6544-6547 (2011)
Daniel I Perez et al.
Bioorganic & medicinal chemistry, 17(19), 6914-6925 (2009-09-15)
Thienylhalomethylketones, whose chemical, biological, and pharmaceutical data are here reported, are the first irreversible inhibitors of GSK-3beta described to date. Their inhibitory activity is likely related to the cysteine residue present in the ATP-binding site, which is proposed as a
Alexandra Vinagre et al.
The journal of adhesive dentistry, 17(2), 107-116 (2015-04-29)
This study compared the microtensile bond strengths (μTBS) of two etch-and-rinse (ER) (OptiBond FL [OBFL]; Prime & Bond NT [PBNT]) and three self-etching (SE) (Clearfil SE Bond [CSEB]; Xeno III [XIII]; Xeno V+ [XV+]) adhesives systems to bur-prepared human enamel
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service