D139505
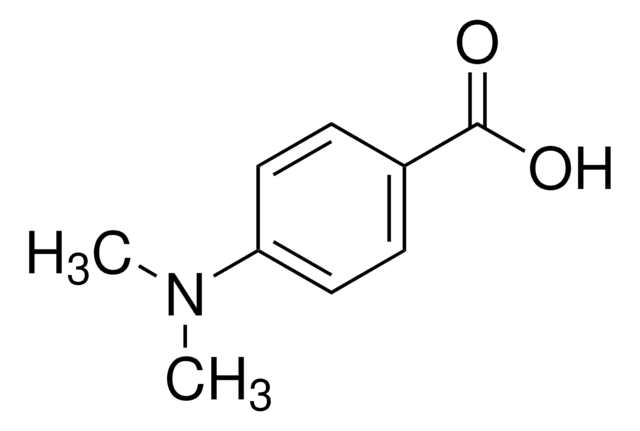
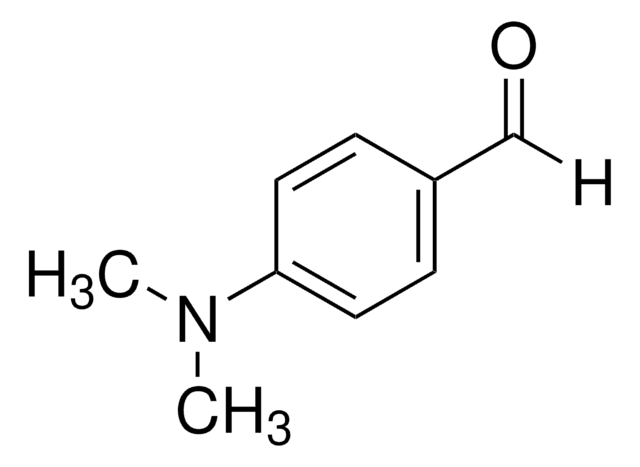
4-(Dimethylamino)benzonitrile
98%
Synonym(s):
4-Cyano-N,N-dimethylaniline, DMABN
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2NC6H4CN
CAS Number:
Molecular Weight:
146.19
Beilstein:
971606
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
bp
318 °C (lit.)
mp
72-75 °C (lit.)
SMILES string
CN(C)c1ccc(cc1)C#N
InChI
1S/C9H10N2/c1-11(2)9-5-3-8(7-10)4-6-9/h3-6H,1-2H3
InChI key
JYMNQRQQBJIMCV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-(Dimethylamino)benzonitrile is extensively used in photophysical studies due to its ability to undergo intramolecular charge transfer (ICT) from the dimethylamino moiety to the cyanophenyl moiety on photo-excitation leading to the appearance of dual fluorescence.
Application
4-(Dimethylamino)benzonitrile can be used in the synthesis of 3,6-diphenyl-2,5-dihydro-pyrrolo[3,4-c]pyrrole-1,4-dione derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Victor A Galievsky et al.
The journal of physical chemistry. A, 115(40), 10823-10845 (2011-08-02)
The excited state behavior of the six m,n-dicyano-N,N-dimethylanilines (mnDCDMA) and m,n-dicyano-(N-methyl-N-isopropyl)anilines (mnDCMIA) is discussed as a function of solvent polarity and temperature. The dicyano moiety in these electron donor (D)/acceptor (A) molecules has a considerably larger electron affinity than the
Comparative chemical mutagenesis; well designed and well assessed. Comparative genetic toxicology: the second UK EMS Collaborative Study.
F H Sobels
Mutation research, 157(2-3), 107-110 (1985-08-01)
Rômulo A Ando et al.
Physical chemistry chemical physics : PCCP, 19(36), 25151-25157 (2017-09-09)
In this work we demonstrate the use of the push-pull model system 4-(dimethylamino)benzonitrile (DMABN) as a convenient molecular probe to investigate the local solvation structure and dynamics by means of time-resolved infrared spectroscopy (TRIR). The photochemical features associated with this
Mohammed H Ahmed et al.
The journal of adhesive dentistry, 21(2), 117-132 (2019-04-06)
Universal adhesives use a combined primer/bonding resin applied either in 2-step etch-and-rinse (2-E&R) or 1-step self-etch (1-SE) mode. This study investigated whether three universal adhesives would benefit from an extra bonding layer (EBL), essentially making them 3-step E&R (3-E&R) and
The formation of a novel mercapturic acid during the metabolism of an N-methyl aromatic amine, 4-cyano-N,N-dimethylaniline.
C J Logan et al.
Biochemical pharmacology, 33(14), 2345-2346 (1984-07-15)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service