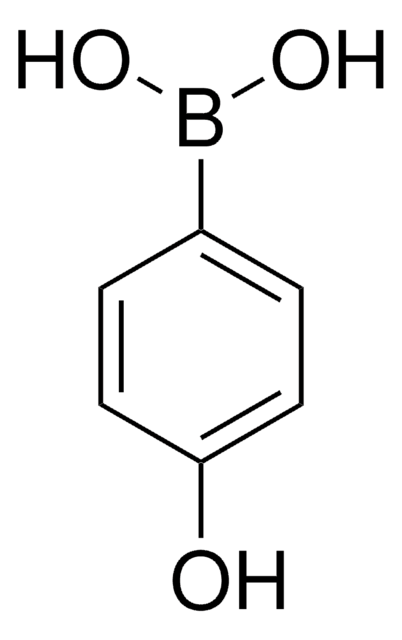
B75956
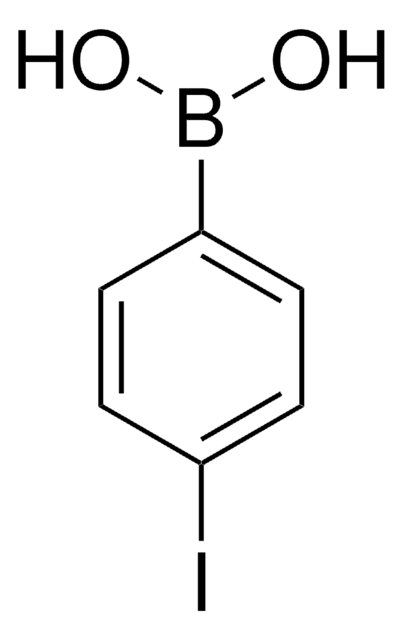
4-Bromophenylboronic acid
≥95.0%
Synonym(s):
(p-Bromophenyl)boronic acid, 4-Bromobenzeneboronic acid, 4-Bromophenylboric acid, p-Bromobenzeneboronic acid, p-Bromophenylboric acid, NSC 25407
About This Item
Recommended Products
Assay
≥95.0%
95%
form
crystals
mp
284-288 °C (lit.)
SMILES string
OB(O)c1ccc(Br)cc1
InChI
1S/C6H6BBrO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4,9-10H
InChI key
QBLFZIBJXUQVRF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Palladium catalyzed Suzuki-Miyaura cross-couplings
- Pd(II)-catalyzed diastereoselective conjugate additions
- Palladium-catalyzed stereoselective Heck-type reaction of allylic esters with arylboronic acids
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides
- Pd-catalyzed arylative cyclization of alkyne-tethered enals or enones via carbopalladation of alkynes
- Copper-catalyzed cross-couplings
Reagent used in Preparation of
- Gallate-based obovatol analogs with potential anti-tumor activity
- Protein modulators and enzymatic and kinase inhibitors
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)