667730
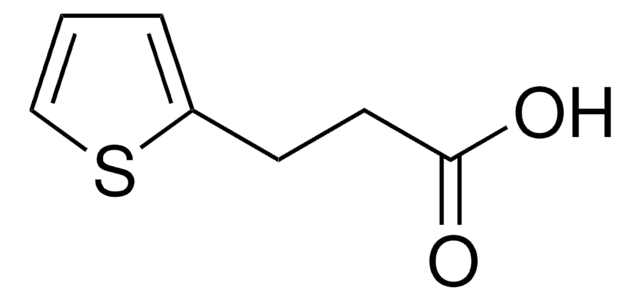
3-(2-Furyl)propionic acid
97%
Synonym(s):
2-Furanpropanoic acid, 2-Furanpropionic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H8O3
CAS Number:
Molecular Weight:
140.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
56-60 °C
functional group
carboxylic acid
SMILES string
OC(=O)CCc1ccco1
InChI
1S/C7H8O3/c8-7(9)4-3-6-2-1-5-10-6/h1-2,5H,3-4H2,(H,8,9)
InChI key
XLTJXJJMUFDQEZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Toshihiro Sato et al.
Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques, 17(4), 475-484 (2015-01-13)
Organic anion-transporting polypeptide (OATP) 1B1 and OATP1B3 contribute to hepatic uptake of numerous drugs. Thus, reduced OATP1B1 and OATP1B3 activity in chronic kidney disease (CKD) may have a major impact on the hepatic clearance of drugs. The effect of drug-uremic
Susanne Kirchhof et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 96, 217-225 (2015-08-09)
Eight-armed PEG was functionalized with furyl and maleimide groups (8armPEG20k-Fur and 8armPEG20k-Mal); degradable hydrogels were obtained by cross-linking via Diels-Alder chemistry. To increase the stability to degradation, the macromonomers were modified by introducing a hydrophobic 6-aminohexanoic acid spacer between PEG
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





gold(I) (2:1) toluene adduct](/deepweb/assets/sigmaaldrich/product/structures/104/897/81ee3e56-c988-4d0f-9614-1269b470316d/640/81ee3e56-c988-4d0f-9614-1269b470316d.png)