62262
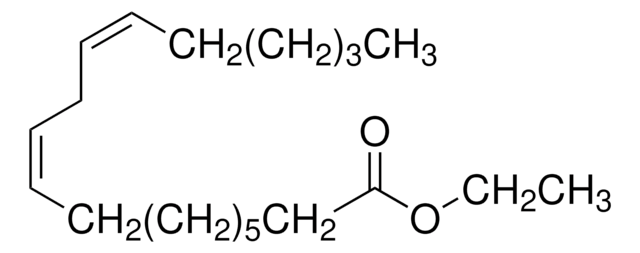
Ethyl linoleate
technical, ≥65% (GC)
Synonym(s):
Linoleic acid ethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)3(CH2CH=CH)2(CH2)7COOC2H5
CAS Number:
Molecular Weight:
308.50
Beilstein:
1727827
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Quality Level
concentration
≥65% (GC)
refractive index
n20/D 1.455 (lit.)
n20/D 1.455
bp
224 °C/17 mmHg (lit.)
density
0.876 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
CCCCC\C=C/C\C=C/CCCCCCCC(=O)OCC
InChI
1S/C20H36O2/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22-4-2/h8-9,11-12H,3-7,10,13-19H2,1-2H3/b9-8-,12-11-
InChI key
FMMOOAYVCKXGMF-MURFETPASA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl linoleate can undergo auto-oxidation in the presence of the catalyst manganese(II)acetylacetonate.
Application
Ethyl linoleate can be used as a drying agent for alkyd paints. It can also form N2,3-ethenoguanine via reaction with deoxyguanosine.
Biochem/physiol Actions
Ethyl linoleate solated from Oxalis triangularis inhibits forskolin-induced melanogenesis and tyrosinase activity in mouse B16 melanoma cells . This anti-melanogenic effect is mediated by inhibiting cAMP production.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
"Fast autoxidation of ethyl linoleate catalyzed by [Mn (acac) 3] and bipyridine: A possible drying catalyst for alkyd paints"
Gorkum VR, et al.
Inorganic Chemistry, 43(08), 2456-2458 (2004)
"4-Hydroxy-2-nonenal and ethyl linoleate form N 2, 3-ethenoguanine under peroxidizing conditions"
Ham.L J-A, et al.
Chemical Research in Toxicology, 1243-1250 (2000)
Yun Ding et al.
Current biology : CB, 29(7), 1089-1099 (2019-03-19)
It is unclear where in the nervous system evolutionary changes tend to occur. To localize the source of neural evolution that has generated divergent behaviors, we developed a new approach to label and functionally manipulate homologous neurons across Drosophila species.
D L Luthria et al.
Biochimica et biophysica acta, 1213(1), 1-4 (1994-06-23)
In order to determine how dietary linoleate is metabolized, rats were maintained on a chemically defined diet containing 1.6% ethyl linoleate. After 5 weeks the linoleate was replaced by an equal amount of ethyl 9,10,12,13-d4-linoleate. The animals were killed 3
H Hara et al.
The Journal of nutrition, 126(4), 800-806 (1996-04-01)
Oxidized ethyl linoleate (OEL) was prepared by aeration at low temperature. Peroxide value (POV, mEq/kg lipid) of OEL was 1400; the major oxidized compounds were 9-hydroperoxy-cis, trans- and 13-hydroperoxy-trans, cis-octadecadienoate. Rats fed fiber-free or sugar-beet fiber (SBF, 100g/kg diet) diets
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service