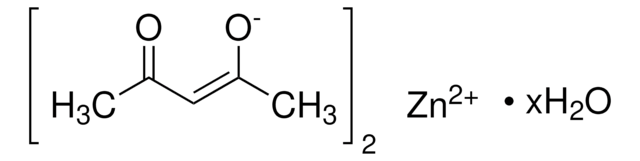
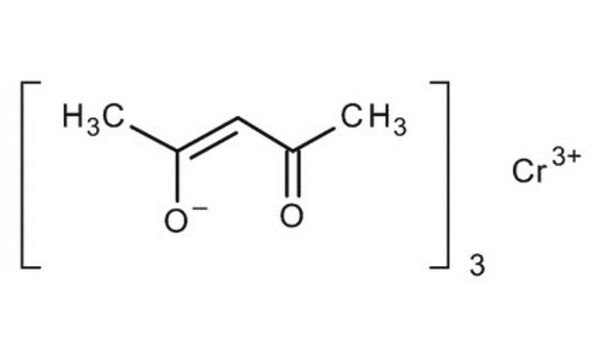
517003
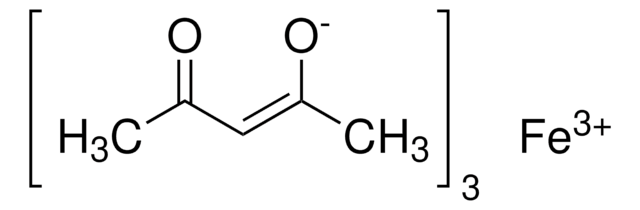
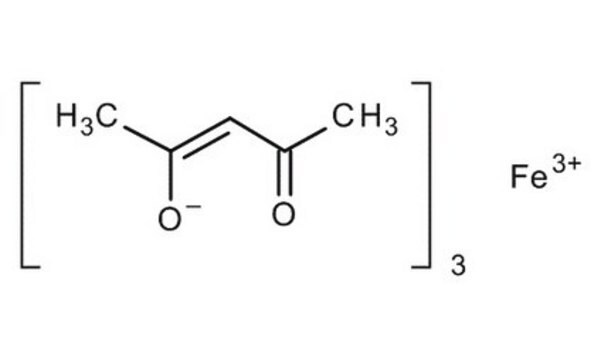
Iron(III) acetylacetonate
≥99.9% trace metals basis
Synonym(s):
2,4-Pentanedione iron(III) derivative, Fe(acac)3, Ferric acetylacetonate, Iron(III) 2,4-pentanedionate
About This Item
Recommended Products
Assay
≥99.9% trace metals basis
form
powder
reaction suitability
core: iron
reagent type: catalyst
mp
180-182 °C (dec.) (lit.)
density
5.24 g/mL at 25 °C (lit.)
SMILES string
CC(=O)\C=C(\C)O[Fe](O\C(C)=C/C(C)=O)O\C(C)=C/C(C)=O
InChI
1S/3C5H8O2.Fe/c3*1-4(6)3-5(2)7;/h3*3,6H,1-2H3;/q;;;+3/p-3/b3*4-3-;
InChI key
AQBLLJNPHDIAPN-LNTINUHCSA-K
Looking for similar products? Visit Product Comparison Guide
General description
Application
- A iron precursor for the synthesis of Fe3O4/carbon composite fibers via forcespinning technique. This composite material used in the formation of high-performance anode materials for lithium-ion batteries.
- A precursor for the synthesis of iron-containing metal-organic frameworks (MOFs) for the applications in rechargeable alkali-ion batteries.
- An additive to enhance the efficiency of the N-hydroxyphthalimide (NHPI) catalyst in the oxidation of cumene.
- A solvent activation agent in the fabrication of polyamide membranes, which are used in reverse osmosis (RO) applications.
- As a MOCVD precursor for highly crystalline (Zn,Fe)Fe2O4 films and magnetic property measurements of these films. Iron (III) acetylacetonate may be used as a precursor for the synthesis of water-soluble magnetite nanoparticles, which may find applications in magnetic hyperthermia treatment.
- As a MOCVD precursor for highly crystalline (Zn,Fe)Fe2O4 films and magnetic property measurements of these films.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Solvothermal synthesis is a method for preparing a variety of materials such as metals, semiconductors, ceramics, and polymers.
Professor Randal Lee (University of Houston, USA) discusses design considerations for iron oxide magnetic nanospheres and nanocubes used for biosensing, including synthetic procedures, size, and shape. The effects of these variables are discussed for various volumetric-based and surface-based detection schemes.
Magnetism and magnetic materials have been of scientific interest for over 1,000 years. More recently, fundamental investigations have focused on exploring the various types of magnetic materials and understanding the magnetic effects created by electric currents.
High Purity Metalorganic Precursors for CPV Device Fabrication
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service