49560
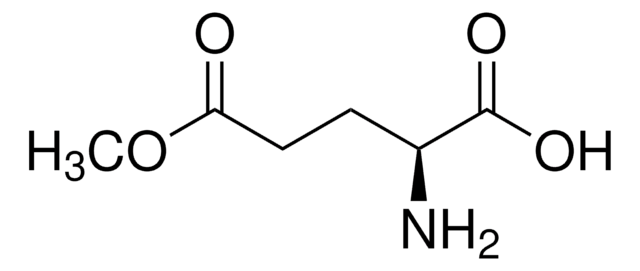
L-Glutamic acid dimethyl ester hydrochloride
≥99.0% (anhydrous basis material, AT)
Synonym(s):
L-Glu(OMe)OMe·HCl
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3COOCH2CH2CH(NH2)COOCH3 · HCl
CAS Number:
Molecular Weight:
211.64
Beilstein:
4238829
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99.0% (anhydrous basis material, AT)
optical activity
[α]20/D +26.0±1°, c = 5% in H2O
reaction suitability
reaction type: solution phase peptide synthesis
impurities
~2% water
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
Cl.COC(=O)CC[C@H](N)C(=O)OC
InChI
1S/C7H13NO4.ClH/c1-11-6(9)4-3-5(8)7(10)12-2;/h5H,3-4,8H2,1-2H3;1H/t5-;/m0./s1
InChI key
MFUPLHQOVIUESQ-JEDNCBNOSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Sener et al.
The American journal of physiology, 267(4 Pt 1), E573-E584 (1994-10-01)
Glutamic acid dimethyl ester (GME; 3.0-10.0 mM) enhanced insulin release evoked by 6.0-8.3 mM D-glucose, 1.0-10.0 mM L-leucine, or 5.0-10.0 mM 2-amino-bicyclo(2,2,1)heptane-2-carboxylic acid, causing a shift to the left of the sigmoidal relationship between insulin output and D-glucose concentration. In
J Rasschaert et al.
Journal of molecular endocrinology, 13(2), 209-217 (1994-10-01)
This study aimed to compare the metabolic and secretory responses of pancreatic islets from animals with non-insulin-dependent diabetes to D-glucose with the effects of the methyl esters of succinic acid (SME) and glutamic acid (GME). The insulin secretory response to
M Joëls et al.
Experimental brain research, 54(3), 455-462 (1984-01-01)
Electrical stimulation of fimbria-fornix (fi-fx) fibers monosynaptically activated many of the neurons tested in the lateral septal complex (LSC) of the rat. The orthodromically activated LSC neurons were classified as "strongly" orthodromically activated (SOA) or "weakly" orthodromically activated ( WOA
J Cancelas et al.
International journal of molecular medicine, 8(5), 531-532 (2001-10-18)
The dimethyl ester of L-glutamic acid (DMG) stimulates insulin release and was proposed as a possible insulinotropic tool in the treatment of non-insulin-dependent diabetes. In such a perspective, it was investigated whether DMG enhances the B-cell secretory response to GLP-1
G N Akoev et al.
Neirofiziologiia = Neurophysiology, 20(4), 457-463 (1988-01-01)
Ampullae of Lorenzini isolated from the skate (Raja clavata) have been investigated in vitro electrophysiologically to determine the nature of the transmitter at the synapse between the electroreceptor cells and afferent fibres. Glutamic acid diethyl ester (GDEE), glutamic acid dimethyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service