446424
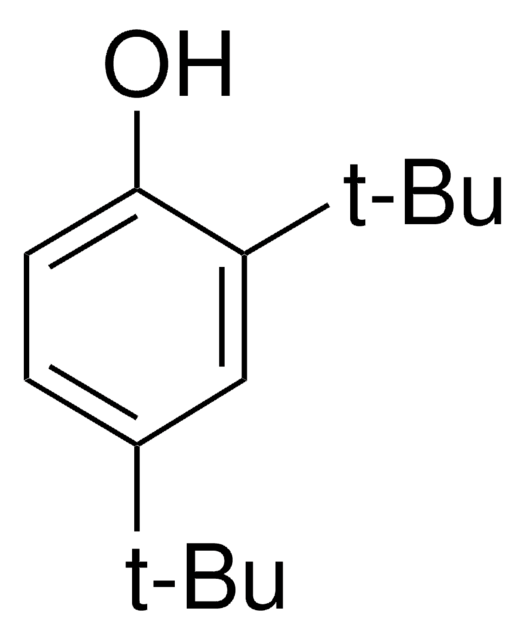
3,5-Di-tert-butyl-4-hydroxybenzyl alcohol
97%
Synonym(s):
3,5-Di-tert-butyl-4-hydroxyphenylmethanol, 4-Hydroxymethyl-2,6-di-tert-butylphenol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOC6H2[C(CH3)3]2CH2OH
CAS Number:
Molecular Weight:
236.35
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
139-141 °C (lit.)
SMILES string
CC(C)(C)c1cc(CO)cc(c1O)C(C)(C)C
InChI
1S/C15H24O2/c1-14(2,3)11-7-10(9-16)8-12(13(11)17)15(4,5)6/h7-8,16-17H,9H2,1-6H3
InChI key
HNURKXXMYARGAY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,5-Di-tert-butyl-4-hydroxybenzyl alcohol can be used as a reactant to synthesize:
- 2,6-di-tert-butyl-4-(dodecylselanylmethyl)phenol and bis(3,5-di-tert-butyl-4-hydroxybenzyl) selenide by reacting with dodecaneselenolate and sodium selenide.
- Monomeric antioxidant by reacting with imidazole and N-[4-(chlorocarbonyl)phenyl]maleimide.
- Sulfur-containing butylated hydroxytoluene derivatives by reacting with aryl/alky dithiols.
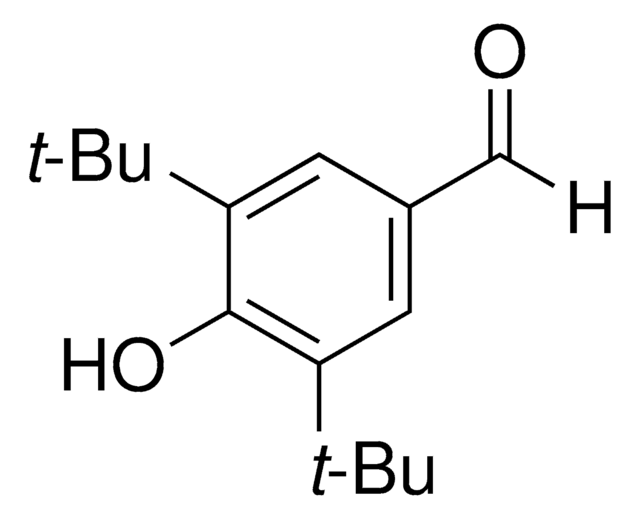
- 3,5-Di-tert-butyl-4-hydroxybenzaldehyde by oxidation reaction using stabilized IBX.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
O L Brekke et al.
Cytokine, 4(4), 269-280 (1992-07-01)
The effect of commonly used food antioxidants on recombinant tumor necrosis factor alpha (rTNF-alpha)-induced cytotoxicity, growth enhancement and adhesion has been evaluated. Butylated hydroxyanisole (BHA) and 4-hydroxymethyl-2,6-di-t-butylphenol (HBP) were the only two of nine antioxidants that completely inhibited rTNF-alpha-induced cytotoxicity
L R Barclay et al.
Biochimica et biophysica acta, 1328(1), 1-12 (1997-08-14)
Phenolic antioxidants of the hydroxychroman class, alpha-tocopherol (alpha-TOC) and 2,2,5,6,7-pentamethyl-6-hydroxychroman (PMHC), and the hindered phenols 2,3-dihydro-5-hydroxy-2,2,4-trimethylnaphtho[1,2-b]furan (NFUR), 2,6-di-tert-butyl-4-methoxyphenol (DBHA), and 2,6-di-tert-butyl-4-methyl phenol (BHT), were delivered into oxidizable (ACCEPTOR) liposomes of dilinoleoylphosphatidylcholine (DLPC) or 1-palmitoyl-2-linoleoyl-phosphatidylcholine (PLPC) from saturated DONOR liposomes of
REACTION OF SEVEN-AND EIGHT-MEMBERED CYCLIC PHOSPHOROCHLORIDITES WITH 3, 5-DI-tert-BUTYL-4-HYDROXYBENZYL ALCOHOL: FACILE P [sbnd] C BOND FORMATION.
Odorisio PA, et al.
Phosph. Sulfur Relat. Elem., 20(3), 273-277 (1984)
The antioxidant activity of 3, 5-di-tert-butyl-4-hydroxybenzyl derivatives.
Kim DH and Kummerow FA.
Journal of the American Chemical Society, 39(3), 150-155 (1962)
Synthesis of new polymeric antioxidants.
Oh DR, et al.
Bull. Korean Chem. Soc., 22(6), 629-632 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service