436372
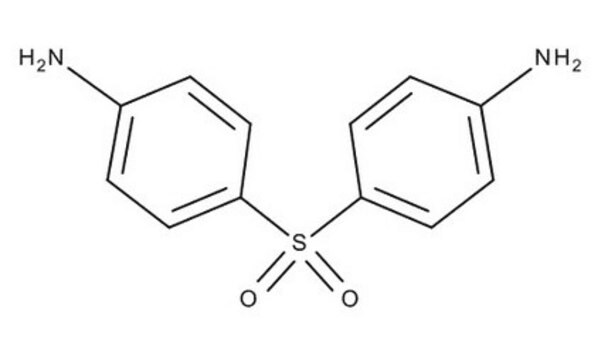
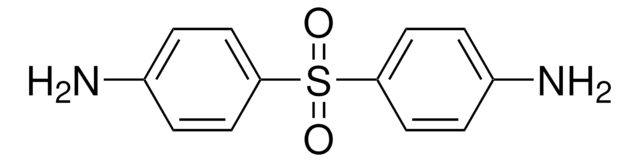
4,4′-Diaminodiphenyl sulfide
98%
Synonym(s):
4,4′-Thiodianiline
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
S(C6H4NH2)2
CAS Number:
Molecular Weight:
216.30
Beilstein:
1875513
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
105-107 °C (lit.)
functional group
thioether
SMILES string
Nc1ccc(Sc2ccc(N)cc2)cc1
InChI
1S/C12H12N2S/c13-9-1-5-11(6-2-9)15-12-7-3-10(14)4-8-12/h1-8H,13-14H2
InChI key
ICNFHJVPAJKPHW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4,4′-Diaminodiphenyl sulfide is a diaminodiphenyl analog. On oral administration to rats, it induces haematological and pathological changes indicative of erythrocyte destruction. It has been reported to generate hydrogen peroxide in erythrocytes in vitro. Enzymatic oxidation of 4,4′-diaminodiphenyl sulfide to their sulfoxide derivatives in guinea pig liver homogenates has been reported.
Application
4,4′-Diaminodiphenyl sulfide may be employed for the fabrication of quantum wires and quantum dots by chemical vapor deposition.
It may be used for the preparation of the following:
It may be used for the preparation of the following:
- polypyromellitimides
- sulfur-containing copolyimides
- polyamides
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Carc. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Quantum wire and dot formation by chemical vapor deposition and molecular layer deposition of one-dimensional conjugated polymer.
Yoshimura T, et al.
Applied Physics Letters, 60(3), 268-270 (1992)
Aromatic polyimides.
Bower GM and Frost LW.
Journal of Polymer Science Part A: Polymer Chemistry, 1(10), 3135-3150 (1963)
R Munday et al.
Journal of applied toxicology : JAT, 5(6), 414-417 (1985-12-01)
Diphenyl disulphide, 4,4'-diaminodiphenyl disulphide, 2,2'-diaminodiphenyl disulphide, 4,4'-dimethyldiphenyl disulphide and 4,4'-dinitrodiphenyl disulphide, when administered orally to rats, induced haematological and pathological changes indicative of erythrocyte destruction in vivo. No evidence of haemolysis was detected, however, in animals receiving diphenyl disulphide-2,2'-dicarboxylic acid
Synthesis and characterization of novel, soluble sulfur-containing copolyimides with high refractive indices.
Dusselberg D, et al.
J. Mater. Sci., 46(14), 4872-4879 (2011)
Polycondensation of pyridine-2,6-dicarboxylic acid with some di-and tetraamino compounds.
Banihashemi A and Eghbali M.
Journal of Polymer Science, 14(11), 2659-2664 (1976)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 436372-25G | 4061832115764 |
| 436372-100G |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service