431982
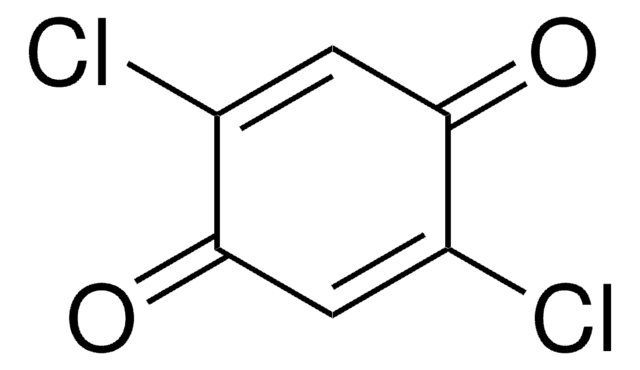
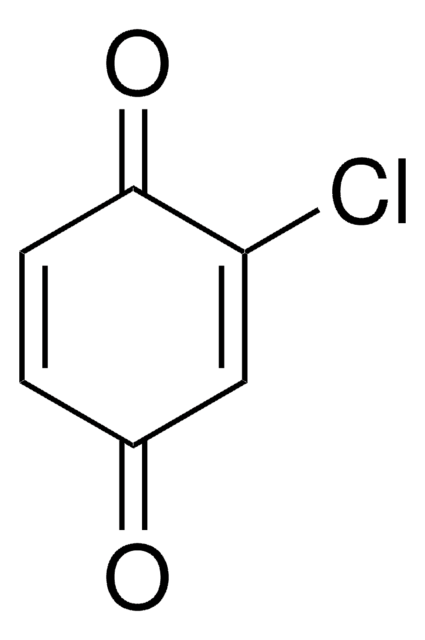
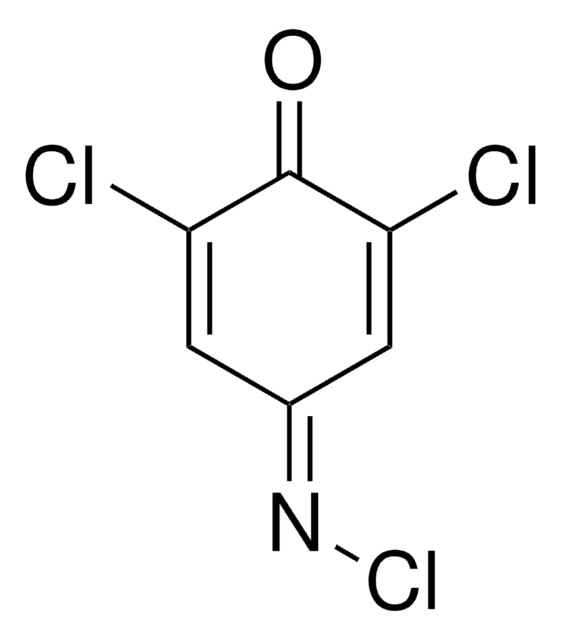
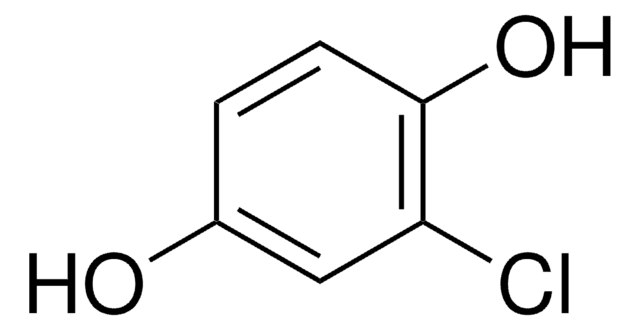
2,6-Dichloro-1,4-benzoquinone
98%
Synonym(s):
2,6-Dichloro-2,5-cyclohexadiene-1,4-dione, 2,6-Dichloro-p-benzoquinone, 2,6-Dichlorobenzoquinone, 2,6-Dichloroquinone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H2Cl2O2
CAS Number:
Molecular Weight:
176.98
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
122-124 °C (lit.)
SMILES string
ClC1=CC(=O)C=C(Cl)C1=O
InChI
1S/C6H2Cl2O2/c7-4-1-3(9)2-5(8)6(4)10/h1-2H
InChI key
JCARTGJGWCGSSU-UHFFFAOYSA-N
Related Categories
General description
2,6-Dichloro-1,4-benzoquinone (DCBQ, 2,6-DCBQ) is a halobenzoquinone. Halobenzoquinones are disinfection byproducts (DBPs) found in drinking water. They are capable of producing reactive oxygen species and causing oxidative damage to proteins and DNA in T24 human bladder carcinoma cells. UV-induced transformation of DCBQ in drinking water has been reported. Detection of DCBQ in drinking water by liquid chromatography-ESI tandem mass spectrometry has been reported.
Application
2,6-Dichloro-1,4-benzoquinone is the suitable reagent used to in a study to evaluate the diffusion coefficients (D) for a family of quinones, nitroaromatics, ferrocenes and aromatic hydrocarbon compounds, in acetonitrile by single potential step chronoamperometry. It may be used in the preparation of :
- 2,3,5-Trichloro-1,4-dihydroquinone
- 2,3,5-trichloro-1,4-benzoquinone
- 2,6-Dichloro-1,4-dihydroquinone
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jinhua Li et al.
Toxicological sciences : an official journal of the Society of Toxicology, 141(2), 335-343 (2014-05-09)
Halobenzoquinones (HBQs) are a new class of drinking water disinfection byproducts (DBPs) and are capable of producing reactive oxygen species and causing oxidative damage to proteins and DNA in T24 human bladder carcinoma cells. However, the exact mechanism of the
Kai He et al.
Journal of hazardous materials, 351, 98-107 (2018-03-10)
Anthropogenic compounds accidentally released to the environment could be important precursors of disinfection byproducts (DBPs) in drinking water treatment processes. In this study, the haloacetic acid formation potentials (HAAFPs) of 155 anthropogenic compounds listed on the Japanese pollutant release and
A Magnuson et al.
Biochemistry, 36(11), 3254-3261 (1997-03-18)
The binding of Mn2+ to manganese-depleted photosystem II was investigated after chemical modification of histidyl and carboxylic acid residues in the presence or absence of the native manganese cluster. K(M) values for Mn2+ were determined from steady-state electron transfer between
Masako Iwai et al.
Photosynthesis research, 87(3), 313-322 (2006-05-16)
PS II-H is a small hydrophobic protein that is universally present in the PS II core complex of cyanobacteria and plants. The role of PS II-H was studied by directed mutagenesis and biochemical analysis in the thermophilic cyanobacterium Thermosynechococcus elongatus
A toxic disinfection by-product, 2,6-dichloro-1,4-benzoquinone, identified in drinking water.
Feng Qin et al.
Angewandte Chemie (International ed. in English), 49(4), 790-792 (2009-12-22)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service