403342
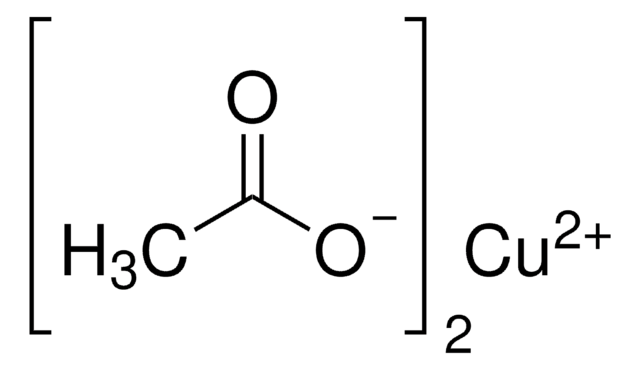
Copper(I) acetate
97%
Synonym(s):
Copper monoacetate, Cuprous acetate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CuCO2CH3
CAS Number:
Molecular Weight:
122.59
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
powder and chunks
reaction suitability
core: copper
reagent type: catalyst
mp
250 °C (dec.) (lit.)
SMILES string
CC(=O)O[Cu]
InChI
1S/C2H4O2.Cu/c1-2(3)4;/h1H3,(H,3,4);/q;+1/p-1
InChI key
RFKZUAOAYVHBOY-UHFFFAOYSA-M
Application
Copper acetate(CuOAc) was used as a starting material for the preparation of copper nanoparticles and CuAlO2 p-type nanostructured semiconductors.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jan Lorkowski et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(48), 11365-11374 (2019-07-06)
Cyclic (amino)(aryl)carbenes (cAArCs) based on the isoindoline core were successfully generated in situ by α-elimination of 3-alkoxyisoindolines at high temperatures or by deprotonation of isoindol-2-ium chlorides with sodium or copper(I) acetates at low temperatures. 3-Alkoxy-isoindolines 2 a,b-OR (R=Me, Et, iPr) have
Qiuyan Jin et al.
European journal of mass spectrometry (Chichester, England), 25(1), 30-43 (2019-02-19)
Gas-phase ion trap mass spectrometry experiments and density functional theory calculations have been used to examine the routes to the formation of the 1,8-naphthyridine (napy) ligated geminally dimetallated phenyl complexes [(napy)Cu2(Ph)]+, [(napy)Ag2(Ph)]+ and [(napy)CuAg(Ph)]+ via extrusion of CO2 or SO2
Room?Temperature Formation of Hollow Cu2O Nanoparticles.
Hung LI, et al.
Advanced Materials, 22.17, 1910-1914 (2010)
Synthesis of delafossite CuAlO 2 p-type semiconductor with a nanoparticle-based Cu (I) acetate-loaded boehmite precursor
Thu TV, et al.
Mat. Res. Bul., 46.11, 1819-1827 (2011)
You Zhai et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 17(5), 741-751 (2015-11-04)
Cu2 S/ZnS heterostructured nanorods (HNRs) with uncommon morphologies are achieved through single-pot and multi-batch synthetic strategies. In both cases, Cu2 S NRs form first, which then undergo partial cation exchange and solution-liquid-solid (SLS)-like growth catalyzed by the remaining Cu2 S
Articles
In this article, we will discuss coinage metal deposition processes in order to provide a sense of the most critical precursors, reducing agents, and processes.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 403342-1G | 4061831986563 |
| 403342-10G | 4061831986556 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service