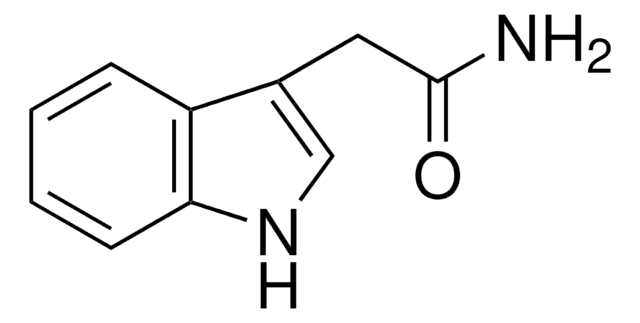
376523
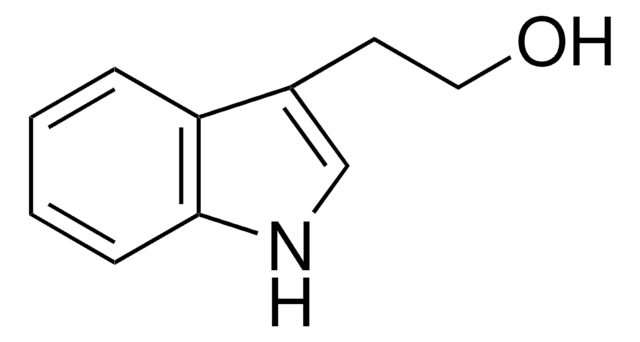
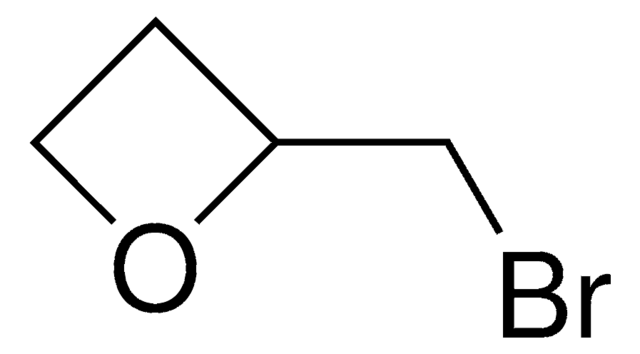
3-(2-Bromoethyl)indole
97%
Synonym(s):
1-Bromo-2-(3-indolyl)ethane, 2-(3-Indolyl)ethyl bromide, 3-(2-Bromoethyl)-1H-indole, 3-(Bromoethyl)-1H-indole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H10BrN
CAS Number:
Molecular Weight:
224.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
97-99 °C (lit.)
solubility
chloroform: soluble 25 mg/mL, clear, yellow
functional group
bromo
SMILES string
BrCCc1c[nH]c2ccccc12
InChI
1S/C10H10BrN/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6H2
InChI key
NTLAICDKHHQUGC-UHFFFAOYSA-N
General description
3-(2-Bromoethyl)indole is a halogenated heterocyclic building block.
Application
3-(2-Bromoethyl)indole may be used in the synthesis of:
- β-carboline derivatives
- 6,7-dihydro-12H-indolo[2,3-a] pyridocolinium bromide
- N-(2-(3-indolyl)ethyl)aza-12-crown-4
- N-(2-(3-Indolyl)ethyl)aza-15-crown-5
- N-(2-(3-Indolyl)ethyl)aza-18-crown-6
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jiaxin Hu et al.
Proceedings of the National Academy of Sciences of the United States of America, 99(8), 5121-5126 (2002-04-12)
Feeble forces play a significant role in the organization of proteins. These include hydrogen bonding, hydrophobic interactions, salt bridge formation, and steric interactions. The alkali metal cation-pi interaction is a force of potentially profound importance but its consideration in biology
The synthesis of ?-carboline derivatives-I: A synthesis of some 12 H-indolo [2, 3-a] pyridocolinium salts, including flavopereirine.
Ban Y and Seo M.
Tetrahedron, 16(1), 5-10 (1961)
The synthesis of beta-carboline derivatives. VII. The isolation of the possible intermediate in the condensation of 3-(2-bromoethyl)indole and 2-halogenopyridine.
Y Ban et al.
Chemical & pharmaceutical bulletin, 13(8), 931-934 (1965-08-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service