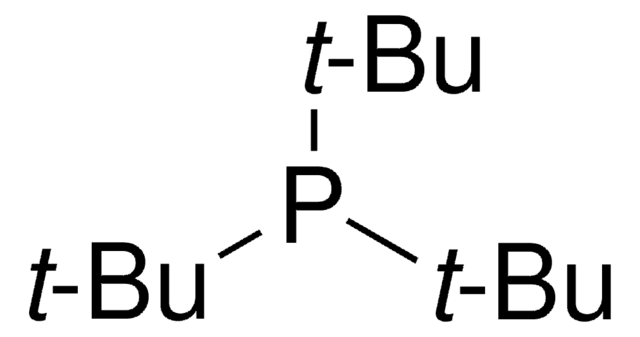366315
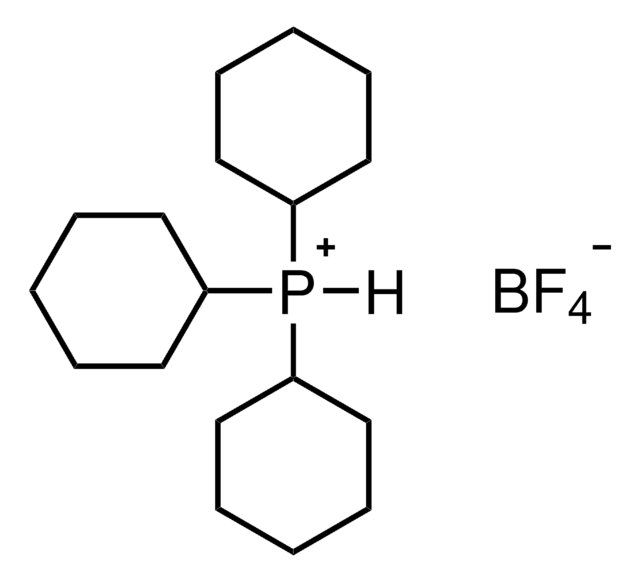
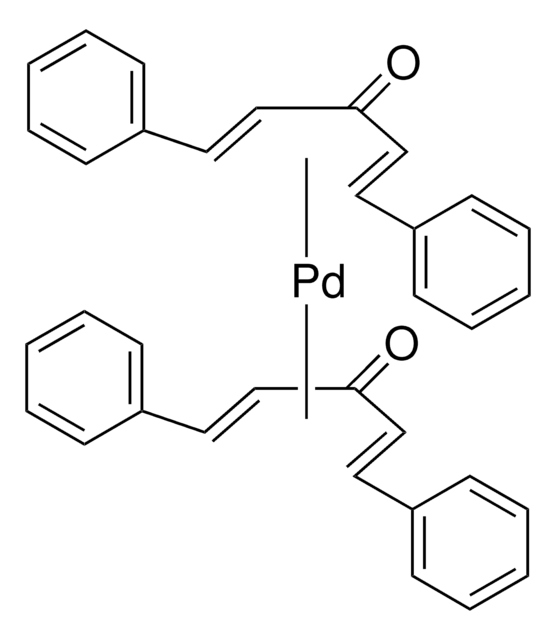
Tris(dibenzylideneacetone)dipalladium(0)-chloroform adduct
Synonym(s):
Dipalladium-tris(dibenzylideneacetone)chloroform complex, Pd2(dba)3 · CHCl3
About This Item
Recommended Products
form
solid
Quality Level
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
131-135 °C (lit.)
SMILES string
[Pd].[Pd].ClC(Cl)Cl.O=C(/C=C/c1ccccc1)\C=C\c2ccccc2.O=C(/C=C/c3ccccc3)\C=C\c4ccccc4.O=C(\C=C\c5ccccc5)/C=C/c6ccccc6
InChI
1S/3C17H14O.CHCl3.2Pd/c3*18-17(13-11-15-7-3-1-4-8-15)14-12-16-9-5-2-6-10-16;2-1(3)4;;/h3*1-14H;1H;;/b3*13-11+,14-12+;;;
InChI key
LNAMMBFJMYMQTO-FNEBRGMMSA-N
General description
Application
- To compose the catalytic system for the preparation of homoallylpalladium complexes.These complexes underwent in situ Stille type cross coupling with various vinyltin reagents to afford the cyclized products bearing allyl appendages.
- As palladium source in the asymmetric transformations of 3,4-epoxy-1-butene.
- As catalyst for the Heck cross-coupling reaction of iodobenzene with styrene.
- As cyclization catalyst.
- As catalyst for [2+2+2] cycloaddtion of didehydrotriphenylenes to the corresponding extended triphenylenes.
- As catalyst for the carbonylation of b,b-imidoyl iodides to the corresponding imidate esters used, in turn, to prepare cyclic, quaternary amino acids.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
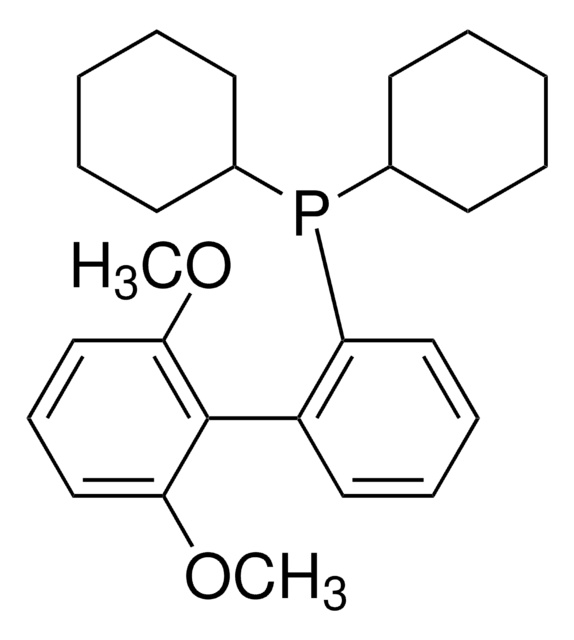
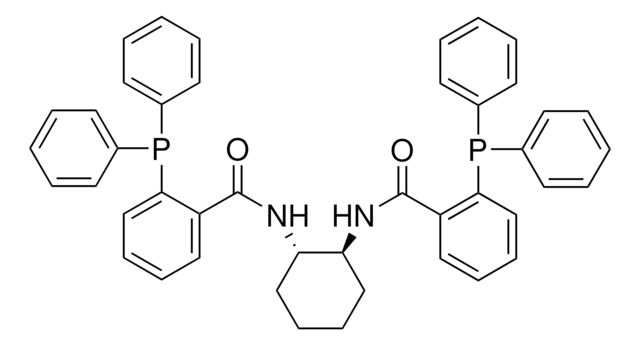
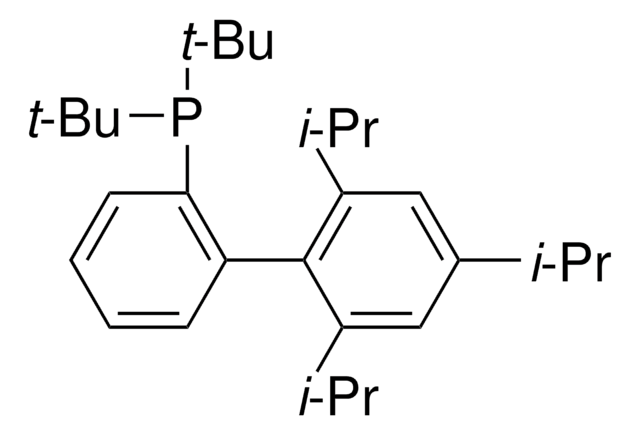
Customers Also Viewed
Articles
A variety of transition-metal catalysts for the Suzuki coupling reaction are now available in our catalog. The majority of these catalysts are palladium- and nickelbased, typically utilizing phosphine-derived ligands.
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 366315-1G | 4061831826050 |
| 366315-250MG | 4061831826067 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)