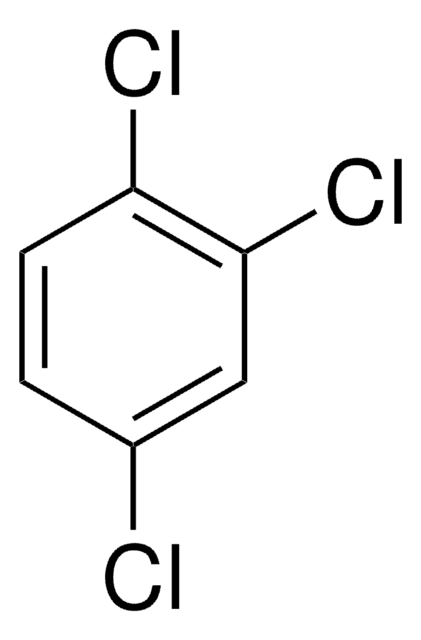
35350
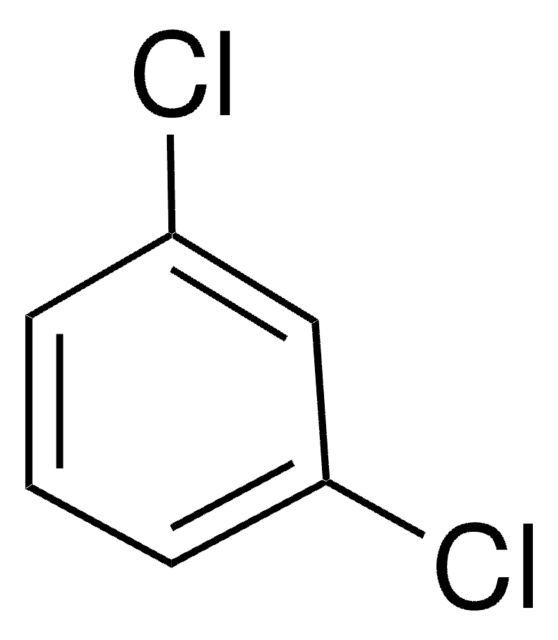
1,3-Dichlorobenzene
puriss., ≥99.0% (GC)
Synonym(s):
m-Dichlorobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H4Cl2
CAS Number:
Molecular Weight:
147.00
Beilstein:
956618
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
5 mmHg ( 38.8 °C)
Quality Level
grade
puriss.
Assay
≥99.0% (GC)
form
liquid
refractive index
n20/D 1.546 (lit.)
n20/D 1.546
bp
172-173 °C (lit.)
mp
−25-−22 °C (lit.)
density
1.288 g/mL at 25 °C (lit.)
functional group
chloro
SMILES string
Clc1cccc(Cl)c1
InChI
1S/C6H4Cl2/c7-5-2-1-3-6(8)4-5/h1-4H
InChI key
ZPQOPVIELGIULI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,3-Dichlorobenzene is a thermodynamically favoured isomer of 1,4-dichlorobenzene. The heat capacity at constant pressure and density of 1,3-dichlorobenzene was measured in the temperature range from (283.15 to 353.15)K.
Application
1,3-Dichlorobenzene is suitable as carbon and energy supplement for the growth of gram-negative, peritrichously flagellated rod, tentatively identified as an Alcaligenes sp.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
152.6 °F - closed cup
Flash Point(C)
67.0 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J Messner et al.
Chemical communications (Cambridge, England), 50(79), 11705-11708 (2014-08-22)
The isomerisation reaction of 1,4-dichlorobenzene leading to the thermodynamically favoured and technically desired 1,3-dichlorobenzene has been studied comparing highly acidic chloroaluminate melts with organic imidazolium and alkali metal ions. Interestingly, the inorganic melts show much higher reactivity and full recyclability
J A de Bont et al.
Applied and environmental microbiology, 52(4), 677-680 (1986-10-01)
A gram-negative, peritrichously flagellated rod, tentatively identified as an Alcaligenes sp., was isolated from a mixture of soil and water samples by using 1,3-dichlorobenzene as the sole carbon and energy source. During growth on 1,3-dichlorobenzene, almost stoichiometric amounts of chloride
Heat capacities and densities of some liquid chloro-, bromo-, and bromochloro-substituted benzenes.
Goralski P and Piekarski H.
Journal of Chemical and Engineering Data, 52(2), 655-659 (2007)
Claudia Amorim et al.
Journal of hazardous materials, 211-212, 208-217 (2011-08-30)
Catalytic hydrodechlorination (HDC) is an effective means of detoxifying chlorinated waste. Involvement of spillover hydrogen is examined in gas phase dechlorination of chlorobenzene (CB) and 1,3-dichlorobenzene (1,3-DCB) over Pd and Ni. The catalytic action of single component Pd and Ni
D Mahadevan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 79(5), 962-969 (2011-05-13)
The FT-IR and FT-Raman vibrational spectra of 1,3-dichlorobenzene (1,3-DCB) have been recorded using Bruker IFS 66 V Spectrometer in the range 4000-100 cm(-1). A detailed vibrational spectral analysis has been carried out and assignments of the observed fundamental bands have
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service