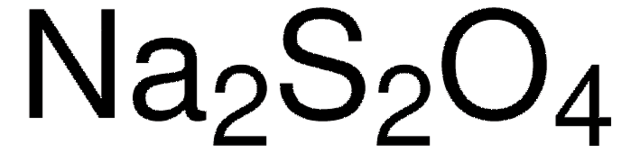336726
Ammonium thiosulfate
98%
Synonym(s):
Diammonium thiosulfate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(NH4)2S2O3
CAS Number:
Molecular Weight:
148.21
EC Number:
MDL number:
UNSPSC Code:
12352302
eCl@ss:
38030427
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
crystals and lumps
mp
150 °C (lit.)
solubility
H2O: very soluble(lit.)
ethanol: insoluble(lit.)
density
1.679 g/mL at 25 °C (lit.)
SMILES string
N.N.OS(O)(=O)=S
InChI
1S/2H3N.H2O3S2/c;;1-5(2,3)4/h2*1H3;(H2,1,2,3,4)
InChI key
XYXNTHIYBIDHGM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ammonium thiosulfate is a non-toxic inorganic compound that is majorly used as a fertilizer and can be used as an alternative to cyanide based leaching. It has a hygroscopic nature and is soluble in water. It can be produced by the reaction of ammonia, water, and sulfur dioxide. It can be used as a photographic fixing salt.
Application
Ammonium thiosulfide can be used as a source of sulfur for the formation of cadmium sulfide thin films for electronic applications. It can also be used in the treatment of copper-gold ores for the recovery of gold.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sulfites, thiosulfates, and dithionites
Barbera JJ, et al.
Ullmann's Encyclopedia of Industrial Chemistry, 66(1-3), 1-21 (2000)
Adsorption of Copper from an Ammonia-Thiosulfate Media Using DOWEX 550A Ion Exchange Resin
Vargas C and Navarro P
International Journal of Nonferrous Metallurgy, 5(04), 33-33 (2016)
The treatment of copper-gold ores by ammonium thiosulfate leaching
Molleman E and Dreisinger D
Hydrometallurgy, 66(1-3), 1-21 (2002)
Electro-plating and characterisation of cadmium sulphide thin films using ammonium thiosulphate as the sulphur source
Abdul-Manaf NA, et al.
Journal of Materials Science: Materials in Electronics, 26(4), 2418-2429 (2015)
Jason A McDonald et al.
Environmental science & technology, 42(2), 398-402 (2008-02-21)
Reducing fumigant emissions is required for minimizing bystander risk and environmental impact. Effective and economic field management methods including commonly used surface sealing technique and soil amendments are needed for achieving emission reductions. This research determined the effectiveness of ammonium
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service