302155
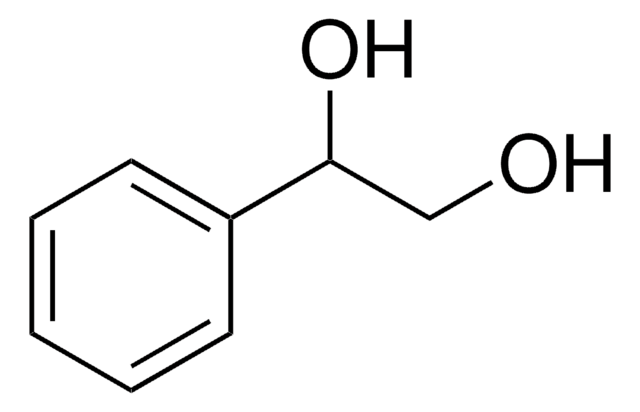
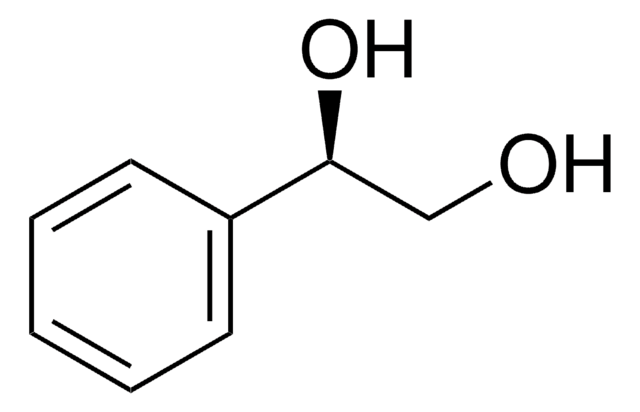
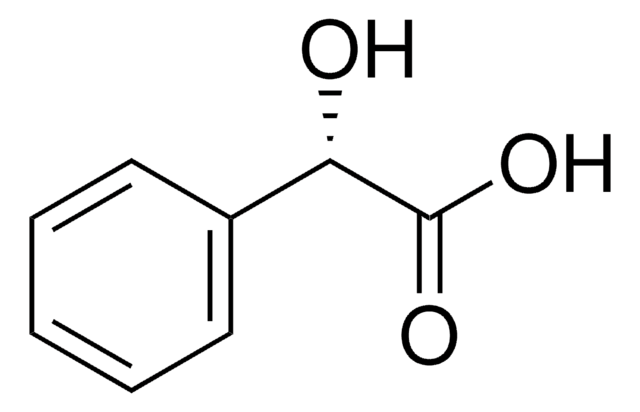
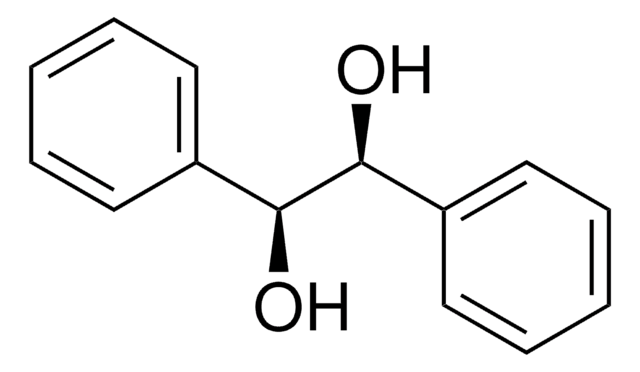
(S)-(+)-1-Phenyl-1,2-ethanediol
99%
Synonym(s):
(+)-Styrene glycol, (S)-(+)-Phenylethylene glycol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOCH2CH(C6H5)OH
CAS Number:
Molecular Weight:
138.16
Beilstein:
3196197
MDL number:
UNSPSC Code:
12352002
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
optical activity
[α]18/D +66°, c = 1 in chloroform
mp
64-67 °C (lit.)
functional group
hydroxyl
phenyl
SMILES string
OC[C@@H](O)c1ccccc1
InChI
1S/C8H10O2/c9-6-8(10)7-4-2-1-3-5-7/h1-5,8-10H,6H2/t8-/m1/s1
InChI key
PWMWNFMRSKOCEY-MRVPVSSYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
320.0 °F - closed cup
Flash Point(C)
160.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jin-Zhao Wang et al.
Biomedical chromatography : BMC, 21(5), 497-501 (2007-03-16)
A simple HPLC method for the simultaneous determination of phenylglyoxylic acid (PGA), mandelic acid (MA), styrene glycol (SG) and hippuric acid (HA) in cell culture medium was developed. Analysis was performed on a C(18) column with a mobile phase composed
Rongzhen Zhang et al.
Wei sheng wu xue bao = Acta microbiologica Sinica, 49(2), 204-209 (2009-05-19)
To prepare (S)-1-phenyl-1,2-ethanediol by a one-step method, we constructed an enzyme coupled system consisting of (R)- and (S)-specific carbonyl reductases in Escherichia coli. The genes coding (R)- and (S)-specific carbonyl reductases from Candida parapsilosis were inserted into a co-expression vector
Li Cao et al.
Biotechnology and bioengineering, 94(3), 522-529 (2006-02-25)
Soluble epoxide hydrolase (EH) from the potato Solanum tuberosum and an evolved EH of the bacterium Agrobacterium radiobacter AD1, EchA-I219F, were purified for the enantioconvergent hydrolysis of racemic styrene oxide into the single product (R)-1-phenyl-1,2-ethanediol, which is an important intermediate
Pablo Taboada et al.
Langmuir : the ACS journal of surfaces and colloids, 21(12), 5263-5271 (2005-06-01)
Three triblock copolymers of ethylene oxide and phenyl glycidyl ether, type E(m)G(n)E(m), where G = OCH2CH(CH2OC6H5) and E = OCH2CH2, were synthesized and characterized by gel-permeation chromatography, matrix-assisted laser desorption ionization time-of-flight mass spectrometry, and NMR spectroscopy. Their association properties
Qingsen Hu et al.
Bioresource technology, 101(21), 8461-8463 (2010-06-25)
In this study, a highly efficient process for Candida parapsilosis-catalyzed deracemization of racemic 1-phenyl-1,2-ethanediol (PED) was described, based on a resin-based in situ substrate feeding and product removal (ISSFPR) methodology. The resin H103 was selected and used to keep the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service