All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H7N
CAS Number:
Molecular Weight:
57.09
Beilstein:
102384
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.432 (lit.)
bp
61-62 °C (lit.)
density
0.847 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C1CNC1
InChI
1S/C3H7N/c1-2-4-3-1/h4H,1-3H2
InChI key
HONIICLYMWZJFZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Phototransformations of azetidine radical cations in freonic matrices under the action of light with λ = 436nm has been investigated. The IR spectrum of azetidine in solid argon matrices has been measured.
Application
Azetidine was employed in:
- a high yielding palladium-catalyzed cross-coupling raection with aryl bromides
- Ullmann type coupling reaction with iodonitroflourenes
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-4.0 °F
Flash Point(C)
-20 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, 243-243 (2007)
Synthesis, 3245-3245 (2006)
Phototransformations of azetidine radical cations stabilized in freonic matrices.
Sorokin ID, et al.
High Energy Chemistry, 48(3), 180-184 (2014)
Experimental and computational studies of the structure and vibrational spectra of azetidine derivatives.
Thompson CA, et al.
Journal of Molecular Structure, 491(1), 67-80 (1999)
Niamh M O'Boyle et al.
European journal of medicinal chemistry, 46(9), 4595-4607 (2011-08-16)
The structure-activity relationships of antiproliferative β-lactams, focusing on modifications at the 4-position of the β-lactam ring, is described. Synthesis of this series of compounds was achieved utilizing the Staudinger and Reformatsky reactions. The antiproliferative activity was assessed in MCF-7 cells
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service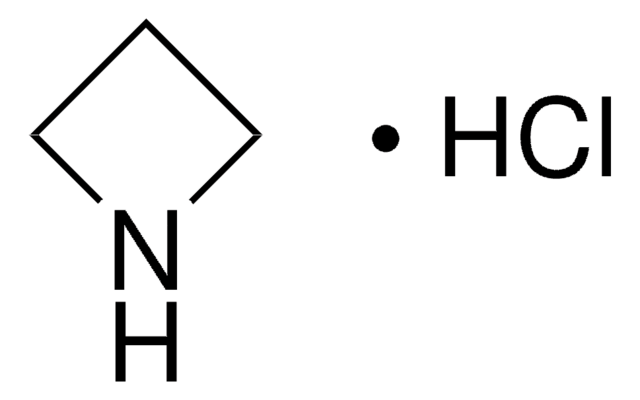
![2-Oxa-6-azaspiro[3.3]heptane](/deepweb/assets/sigmaaldrich/product/structures/391/874/ff74bb51-dd44-4cca-9b3f-3b380ccae360/640/ff74bb51-dd44-4cca-9b3f-3b380ccae360.png)