270822
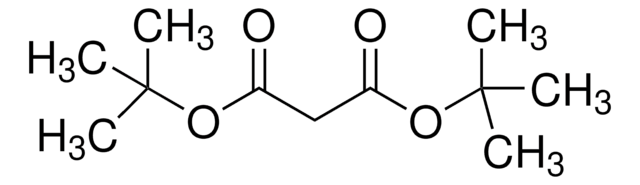
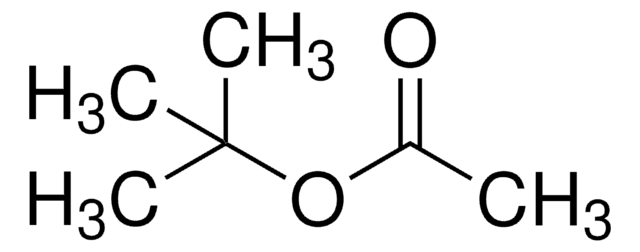
Di-tert-butyl acetylenedicarboxylate
98%
Synonym(s):
2-Butynedioic acid di-tert-butyl ester, Di-tert-butyl 2-butynedioate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)3COCOC≡CCOOC(CH3)3
CAS Number:
Molecular Weight:
226.27
Beilstein:
1957547
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
80-82 °C/0.05 mmHg (lit.)
mp
33-37 °C (lit.)
functional group
ester
SMILES string
CC(C)(C)OC(=O)C#CC(=O)OC(C)(C)C
InChI
1S/C12H18O4/c1-11(2,3)15-9(13)7-8-10(14)16-12(4,5)6/h1-6H3
InChI key
FBCRUXRGQFLOMC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The cross-cyclotrimerization of di-tert-butyl acetylenedicarboxylate, silylacetylenes and acrylamides was studied with cationic rhodium(I)/(R)-tol-binap complex as catalyst. Glycosyl azides were subjected to 1,3-dipolar cycloaddition with di-tert-butyl acetylenedicarboxylate.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
W Bröder et al.
Carbohydrate research, 249(1), 221-241 (1993-10-18)
Glycosyl azides provide reliable anomeric protection stable to conditions for hydrolytic removal of ester groups, for reductive opening or release of acetalic diol protection, for the introduction of ether-type protection, and for glycosylation processes. The utility of this anomeric protection
Jun Hara et al.
Angewandte Chemie (International ed. in English), 53(11), 2956-2959 (2014-02-08)
It has been established that a cationic rhodium(I)/(R)-tol-binap complex catalyzes the cross-cyclotrimerization of silylacetylenes, di-tert-butyl acetylenedicarboxylates, and acrylamides with excellent chemo-, regio-, and enantioselectivities. Unsymmetrical alkynoates can also be employed in place of di-tert-butyl acetylenedicarboxylate for this process, but with
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 270822-5G | 4061832618821 |
| 270822-1G | 4061825922263 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service